Summary
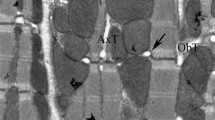
The polyene compound, filipin, was used as a probe to localize cholesterol in the membranes of the rat cardiac muscle cell, with particular reference to the sarcoplasmic reticulum (SR). Filipin binds specifically to cholesterol (and related 3-β-hydroxysterols) in membranes, producing distinct deformations which can be viewed by freeze-fracture and used as markers for the presence of cholesterol-rich regions in the membrane plane. In freeze-fracture replicas of filipin-treated rat myocardium, the muscle cells revealed abundant deformations in their plasma membranes, no deformations in mitochondrial membranes, and an intermediate response in the SR. These results are in agreement with the levels of cholesterol reported in isolated fractions of the different membrane types, and confirm the specificity of filipin action. Within the SR, the filipin-induced deformations were not randomly distributed but occurred more commonly in free SR at or near the Z-region of the sarcomere than in other parts of the free SR or the junctional SR. This finding is interpreted as evidence for a non-homogeneous distribution of cholesterol in cardiac muscle cell SR. The possible significance of cholesterol in relation to structural differentiation and function of the SR is discussed.
Similar content being viewed by others
References
Bittman R (1978) Sterol-polyene antibiotic complexation: probe of membrane structure. Lipids 13:686–691
Branton D, Bullivant S, Gilula NB, Karnovsky MJ, Moor H, Mühlethaler K, Northcote DH, Packer L, Satir B, Satir P, Speth V, Staehelin LA, Steere RL, Weinstein RS (1975) Freeze-etching nomenclature. Science 190:54–56
Bray DF, Rayns DG, Wagenaar EB (1978) Intramembrane particle densities in freeze-fractured sarcoplasmic reticulum. Canad J Zool 56:140–145
Coleman R (1968) Some features of the lipid composition of rat liver surface and cytoplasmic membranes. Chem Phys Lipids 2:144–146
Deamer DW, Baskin RJ (1969) Ultrastructure of sarcoplasmic reticulum preparations. J Cell Biol 42:296–307
De Kruijff B, Gerritsen WJ, Oerlemans A, Van Dijk PWM, Demel RA, Van Deenen LLM (1974) Polyene antibiotic-sterol interactions in membranes of Acholeplasma laidlawii cells and lecthin liposomes. II. Temperature dependence of the polyene antibiotic-sterol complex formation. Biochim Biophys Acta 339:44–56
Demel RA, De Kruyff B (1976) The function of sterols in membranes. Biochim Biophys Acta 457:109–132
Ebashi S, Endo M (1968) Calcium ion and muscle contraction. Prog Biophys Mol Biol 18:123–183
Elias PM, Friend DS, Goerke J (1979) Membrane sterol heterogeneity. Freeze-fracture detection with saponins and filipin. J Histochem Cytochem 27:1247–1260
Fiehn W, Peter JB, Mead JF, Gan-Elapano M (1971) Lipids and fatty acids of sarcolemma, sarcoplasmic reticulum and mitochondria from rat skeletal muscle. J Biol Chem 246:5617–5620
Franzini-Armstrong C (1975) Membrane particles and transmission at the triad. Fed Proc 34:1382–1389
Friend DS, Havel CM, Silberklang M, Watson JA (1980) Distribution and effects of cholesterol on 3-β-hydroxysterol-free eukaryotic cells. J Cell Biol 87:196a
Headon DR, Barrett EJ, Joyce NM, O'Flaherty J (1977) Cholesterol in muscle membranes. Mol Cell Biochem 17:117–123
Heusson-Stiennon J-A, Wanson JC, Drochmans P (1972) Isolation and characterization of the sarcoplasmic reticulum of skeletal muscle. J Cell Biol 55:471–488
Jain MH (1975) Role of cholesterol in biomembranes and related systems. Curr Top Membr Transp 6:1–57
Kitajima Y, Sekiya T, Nozawa Y (1976) Freeze-fracture ultrastructural alterations induced by filipin, pimaricin, nystatin and amphotericin B in the plasma membranes of Epidermophyton, Saccharomyces and red blood cells. A proposal for polyene-ergosterol complex-induced membrane lesions. Biochim Biophys Acta 445:452–465
Klein I, Moore L, Pastan I (1978) Effect of liposomes containing cholesterol on adenylate cyclase activity of cultured mammalian fibroblasts. Biochim Biophys Acta 506:42–53
Lau YH, Caswell AH, Brunschwig J-P, Baerwald RJ, Garcia M (1979) Lipid analysis and freeze-fracture studies on isolated transverse tubules and sarcoplasmic reticulum subfractions of skeletal muscle. J Biol Chem 254:540–546
MacLennan DH (1975) Resolution of the calcium transport system of sarcoplasmic reticulum. Canad J Biochem 53:251–261
Madden TD, Chapman D, Quinn PJ (1979) Cholesterol modulates activity of calcium-dependent ATPase of the sarcoplasmic reticulum. Nature 279:538–541
Mas-Oliva J (1980) The calcium transporting properties of the cardiac muscle cell membrane. PhD Thesis, University of London
Mathias RT, Levis RA, Eisenberg RS (1980) Electrical models of excitation-contraction coupling and charge movement in skeletal muscle. J Gen Physiol 76:1–31
Meissner G (1975) Isolation and characterization of two types of sarcoplasmic reticulum vesicles. Biochim Biophys Acta 389:51–68
Montesano R, Perrelet A, Vassalli P, Orci L (1979) Absence of filipin-sterol complexes from large coated pits on the surface of culture cells. Proc Natl Acad Sci USA 76:6391–6395
Montesano R, Vassalli P, Perrelet A, Orci L (1980) Distribution of filipin-cholesterol complexes at sites of exocytosis — a freeze-fracture study of degranulating mast cells. Cell Biol Int Rep 4:975–984
Morré DJ (1977) The Golgi apparatus and membrane biogenesis. In: Poste G, Nicolson GL (eds) The synthesis, assembly and turnover of cell surface components. Cell Surface Reviews Vol 4. ElsevierNorth-Holland Biomedical Press, Amsterdam, p 1–83
Nakajima Y, Bridgman PC (1981) Absence of filipin-sterol complexes from the membranes of active zones and acetylcholine receptor aggregates at frog neuromuscular junctions. J Cell Biol 88:453–458
Orci L, Miller RG, Montesano R, Perrelet A, Amherdt M (1980 a) Opposite polarity of filipin-induced deformations in the membrane of condensing vacuoles and zymogen granules. Science 210:1019–1021
Orci L, Montesano R, Brown D (1980 b) Heterogeneity of toad bladder granular cell luminal membranes. Distribution of filipin-sterol complexes in freeze-fracture. Biochim Biophys Acta 601:443–452
Orci L, Montesano R, Meda P, Malaisse-Lagae F, Brown D, Perrelet A, Vassalli P (1981 a) Heterogeneous distribution of filipin-cholesterol complexes across the cisternae of the Golgi apparatus. Proc Natl Acad Sci USA 78:293–297
Orci L, Singh A, Amherdt M, Brown D, Perrelet A (1981 b) Microheterogeneity of protein and sterol content in kidney podocyte membrane. Nature 293:646–647
Podolsky RJ, Hall T, Hatchett SL (1970) Identification of oxalate precipitates in striated muscle fibres. J Cell Biol 44:699–702
Robenek H, Greven H (1981) Freeze-fracture evidence for high cholesterol content in nuclear membranes of a larval urodelan epidermis. Eur J Cell Biol 25:131–135
Robenek H, Jung W, Gebhardt R (1982) The topography of filipin-cholesterol complexes in the plasma membrane of cultured hepatocytes and their relation to cell junction formation. J Ultrastruct Res 78:95–106
Robinson JM, Karnovsky MJ (1980) Evaluation of the polyene antibiotic filipin as a cytochemical probe for membrane cholesterol. J Histochem Cytochem 28:161–168
Ryan DM, Shafiq SA (1980) A freeze-fracture study of the anterior and posterior latissimus dorsi muscles of the chicken. Anat Rec 198:147–161
Sekiya T, Kitajima Y, Nozawa Y (1979) Effects of lipid phase separation on the filipin action on membranes of ergosterol-replaced Tetrahymena cells, as studied by freeze-fracture electron microscopy. Biochim Biophys Acta 550:269–278
Severs NJ (1981 a) Freeze-fracture detection of cholesterol in filipin-treated cardiac muscle cell membranes. J Mol Cell Cardiol 13 (Suppl) 1:86
Severs NJ (1981 b) Plasma membrane cholesterol in myocardial muscle and capillary endothelial cells. Distribution of filipin-induced deformations in freeze-fracture. Eur J Cell Biol 25:289–299
Severs NJ (1981 c) Localization of cholesterol in the Golgi apparatus of cardiac muscle cells. Experientia 37:1195–1198
Severs NJ, Warren RC, Barnes SH (1981) Analysis of membrane structure in the transitional epithelium of rat urinary bladder. 3. Localization of cholesterol using filipin and digitonin. J Ultrastruct Res 77:160–188
Sommer JR, Johnson EA (1979) Ultrastructure of cardiac muscle. In: Berne RM, Sperelakis N, Geiger SR (eds) Handbook of Physiology Section 2: The cardiovascular system, Vol 1, The heart. American Physiological Society, Bethesda Maryland, p 113–186
Sommer JR, Waugh RA (1976) The ultrastructure of the mammalian cardiac muscle cell —with special emphasis on the tubular membrane systems. Am J Pathol 82:192–211
Sommer JR, Dolber PC, Taylor I (1980 a) Filipin-cholesterol complexes in the sarcoplasmic reticulum of frog skeletal muscle. J Ultrastruct Res 72:272–285
Sommer JR, Wallace NR, Junker J (1980 b) The intermediate cisterna of the sarcoplasmic reticulum of skeletal muscle. J Ultrastruct Res 71:126–142
Tillack TW, Kinsky SC (1973) A freeze-etch study of the effects of filipin on liposomes and human erythrocyte membranes. Biochim Biophys Acta 323:43–54
Verkleij AJ, De Kruijff B, Gerritsen WF, Demel RA, Van Deenen LLM, Ververgaert PHJ (1973) Freeze-etch electron microscopy of erythrocytes, Acholeplasma laidlawii cells and liposomal membranes after the action of filipin and amphotericin B. Biochim Biophys Acta 291:577–581
Winegrad S (1965) Autoradiographic studies of intracellular calcium in frog skeletal muscle. J Gen Physiol 48:455–479
Winegrad S (1970) The intracellular site of calcium activation of contraction in frog skeletal muscle. J Gen Physiol 55:77–88
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Severs, N.J. Cholesterol distribution and structural differentiation in the sarcoplasmic reticulum of rat cardiac muscle cells. Cell Tissue Res. 224, 613–624 (1982). https://doi.org/10.1007/BF00213756
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00213756