Abstract
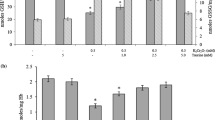
The toxicity of iron (II) and iron (III) chlorides was studied at different biochemical and cellular levels, including antioxidative and metabolic enzymes and two general indicators of cytotoxicity in Vero monkey kidney cells after 24-h exposure. Iron (II) was fourfold more toxic than Fe (III) in cell proliferation, with EC50 of 5.5 and 22 mM, respectively. Metabolic markers were far more sensitive than cytotoxicity assays at these concentrations. At the highest concentrations of toxicant tested [10 mM Fe(II) and 50 mM Fe (III)], both species produced nearly total inhibition of the relative uptake of neutral red (RNRU) and phosphofructokinase activity (PFK), and stimulated intracellular specific lactate dehydrogenase activity (LDH). Succinate dehydrogenase (SDH) and hexosaminidase (HEX) activities were reduced in a dose-dependent manner, as was the antioxidative enzyme glucose-6-phosphate dehydrogenase (G-6-PDH) with both forms of iron. Glutathione reductase (GOR) and glutathione-S-transferase (GST) activities were stimulated by Fe (II) but were inhibited by the higher Fe (III) concentrations. In conclusion, the experimental model may be useful for the study of different metabolic effects induced by the two oxidation states of iron.
Similar content being viewed by others
References
Andres MI (1994) Evaluación in vitro de la citotoxicidad y neurotoxicidad de compuestos orgánicos. PhD Thesis, Universidad de Sevilla
Aust SD, Svingen BA (1982) The role of iron in enzymatic lipid peroxidation. In: Pryor WA (ed) Free Radicals in Biology, vol 5. Academic Press, NY, pp 1–28
Baggiolini M, DeDuve C, Masson PL, Heremans JF (1970) Association of lactoferrin with specific granules in rabbit heterophil leukocytes. J Exp Med 131:559–564
Bradford M (1976) A rapid sensitive method for quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Brigelius R (1985) Mixed disulfides: biological functions and increase in oxidative stress. In: Sies H (ed) Oxidative Stress. Academic Press, London, pp 243–272
Das B, Khtoon N, Shrivastava RC, Viswanathan PN, Rhaman Q (1983) Biochemical studies on the toxicity of hematite dust. Environ Res 32:372–381
Duffy PA, Flint OP (1987) In vitro dermal irritance test. In: Atterwill CK, Steel CE (eds) In Vitro Methods in Toxicology. Cambridge University Press, Cambridge, pp 279–297
Elinder CG (1986) Iron. In: Friberg L, Nordeberg GF, Vouk VB (eds) Handbook on the Toxicology of Metals, vol 2, Second edition. Elsevier, Amsterdam, pp 276–297
García-Alfonso C, Martínez-Galisteo E, Llobell A, Bárcena JA, López-Barea J (1993) Regulation of horse-liver glutathione reductase. Int J Biochem 25:513–520
García-Alfonso C, López-Barea J, Sanz P, Repetto G, Repetto M (1995a) Stimulation of antioxidative enzymes by paraquat in cultured Vero cells. Vet Hum Toxicol 37:414–421
García-Alfonso C, Sanz P, Repetto G, Repetto M, López-Barea J (1995b) Direct determination of glutathione reductase in cells cultured in microtitre plates as a biomarker for oxidative stress. ATLA 23:531–538
Habig WH, Pabst MJ, Jakoby WB (1974) Glutathione-S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem 249:7130–7139
Haliwell B, Gutteridge JMC (1984) Role of iron in oxygen radical reactions. Methods Enzymol 105:47–56
—, — (1991) Role of free radicals and catalytic metal ions in human desease; an overview. Methods Enzymol 186:1–85
Hann WD, Rhim JS (1974) Vero (Kidney, African Green Monkey, Cercopitecus aethiops). In: Flow manual. Flow Laboratories Ltd, Irvine, UK, pp 4–45
Hentze WH (1995) Post-transcriptional regulation of gene expresion by iron. In: Baeuerle PA (ed) Inducible Gene Expresión vol 1: Environmental Stresses and Nutrients. Birkhäuser. Boston, pp 241–265
Hersko C (1989) Mechanism of iron toxicity and its possible role in red cell membrane damage. Semin Hematol 26:277–285
Kornberg A, Horecker BL (1955) Glucose-6-phosphate dehydrogenase. Enzymes of carbohidrate metabolism. Methods Enzymol 1:324–324
Landegren U (1984) Measurement of cell number by means of the endogenous enzyme hexosaminidase. Applications to detection of lymphocytes and cell surface antigens. J Immunol Methods: 67:379–388
Lawlor SM, O'Brien NM (1994) Development of an in vitro cell culture model to investigate the induction and quantification of oxidative stress and its inhibition by α-tocopherol. Toxicol in Vitro: 8:67–73
Ling KH, Paetkau V, Marcus F, Lardy HA (1966) Phosphofructokinase (I. Skeletal muscle). Methods Enzymol 9:425–426
Llobell A, López-Ruiz A, Peinado J, López-Barea J (1988) Glutathione reductase directly mediates the stimulation of yeast glucose-6-phosphate dehydrogenase by GSSG. Biochem J 249:293–296
Masanet J, Gómez-Lechón MJ, Castell JV (1988) Hepatic toxicity of paraquat in primary cultures of rat hepatocytes. Toxicol in Vitro 2:275–282
Morehouse LA, Tien M, Bucher JR, Aust AD (1983) Effect of hydrogen peroxide on the initiation of microsomal lipid peroxidation. Biochem Pharmacol 32:123–127
Morel I, Lescoat G, Cillard J, Pasdeloup N, Brissot P, Cillard P (1990) Kinetic evaluation of free malondialdehyde and enzyme leakage as indices of iron damage in rat hepatocyte cultures. Involvement of free radicals. Biochem Pharmacol 39:1647–1655
Mossman T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65:55–63
Peinado J, Florindo J, López-Barea J (1992) Glutathione reductase from Saccharomyces cerevisiae undergoes redox interconversion in situ and in vivo. Mol Cell Biochem 110:135–143
Pinto MC, Mata AM, López-Barea J (1984) Reversible inactivation of Saccharomyces cerevisiae glutathione reductase under reducing conditions. Arch Biochem Biophys 228:1–12
Repetto G, Sanz P (1993) Neutral red uptake, cellular growth and lysosomal function: in vitro effects of 24 metals. ATLA 21:501–507
Repetto G, Sanz P, Repetto M (1994) Comparative in vitro effects of sodium arsenite and sodium aresenate on neuroblastoma cells. Toxicology 92:143–153
Rodríguez-Ariza A, Martínez-Lara E, Pascual P, Pedrajas JR, Abril N, Dorado G, Toribio F, Bárcena JA, Peinado J, Pueyo C, López-Barea J (1993a) Biochemical and genetic indices of marine pollution in Spanish littoral. Sci Total Environ (Suppl. 1993):109–116
Rodríguez-Ariza A, Peinado J, Pueyo C, López-Barea J (1993b) Biochemical indicators of oxidative stress in fish from polluted littoral areas. Can J Fish Aquat Sci 50:2568–2573
Sies H (1986). Biochemistry of oxidative stress. Angew Chem Int Ed Engl 25:1058–1071
Sokal RR, Rohlf FJ (1969) Biometry. WH Freeman, NY, pp 1–832
Spivey Fox MR, Rader JI (1988) Iron. In: Seiler HG, Sigel A, Sigel H (eds) Handbook on Toxicity of Inorganic Compounds. Marcel Dekker, NY, pp 345–358
Stevens TM, Boswell GA Jr, Adler R, Ackerman NR, Kerr JS (1988) Induction of antioxidant enzymes activities by a phenilurea derivative, EDU. Toxicol Appl Pharmacol 96:33–42
Trenti T, Botti B, Dessi MA, Predieri G, Masini A (1986) Stuctural and functional properties of rat liver mitochondria in experimental iron overload: iron accumulation and lipoperoxidation. IRCS Med Sci 14:837–838
Winterbourn CC, Vile GF, Monteiro HP (1991) Ferritin, lipid peroxidation and redox-cycling xenobiotics. Free Rad Res Comms 12–13:107–114
Witzleben CL, Chaffey NY (1966) Acute ferrous sulfate poisoning. Arch Pathol 82:454–459
Zager RA, Schimpf BA, Bredl CR, Gmur DJ (1993) Inorganic iron effects on in vitro hypoxic proximal renal tubular cell injury. J Clin Invest 91:702–708
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
García-Alfonso, C., López-Barea, J., Sanz, P. et al. Changes in antioxidative activities induced by Fe (II) and Fe (III) in cultured vero cells. Arch. Environ. Contam. Toxicol. 30, 431–436 (1996). https://doi.org/10.1007/BF00213392
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00213392