Abstract
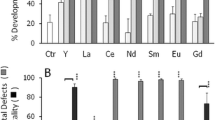
The present study was undertaken to evaluate the toxicity of aluminum sulfate, ferric chloride and their 1:1 mixture (Mix) on early development, fertilization and offspring quality in three sea urchins species (Sphaerechinus granularis, Paracentrotus lividus, Psammechinus microtuberculatus) and in mussels (Mytilus galloprovincialis). The endpoints were the following: a) larval malformations; b) developmental arrest; c) embryonic mortality; d) fertilization success; e) cytogenetic effects, and f) luminol-dependent chemiluminescence (LDCL). Overall data point to the induction of developmental defects in both sea urchin and mussel embryos following exposure of embryos to Al(III) or Fe(III) (10−7 to 10−6 M), whereas Mix caused varied effects vs. Al(III) or Fe(III) alone, from scarce or no additive effects (M. galloprovincialis and P. lividus) to a dramatic rise in embryolethality even at nominal levels of 10−8 M (Ps. microtuberculatus). S. granularis sperm underwent a dose-dependent decrease in fertilization success following exposure to Al(III), or Fe(III), or Mix at levels ranging from 10−8 to 10−5 M. A significant increase of developmental defects was observed in the offspring of S. granularis sperm exposed to micromolar levels of the agents, suggesting an Al(III)- and Fe(III)-related transmissible damage to sperm. The cytogenetic analysis of Al(III)-, Fe(III)-, or Mix-exposed S. granularis embryos showed a significant increase in mitotic aberrations. A relevant feature of the observed cytogenetic damage included scattered chromosomes, suggesting cytoskeleton damage. The LDCL emission in S. granularis embryos showed a dose-related inhibition by agent levels ranging from 10−7 to 10−5 M; this held true for both spontaneous and, to a larger extent, for horseradish peroxidase (HRP)-activated LDCL. LDCL associated with fertilization was affected by Al(III), Fe(III) and Mix, with a time- and dose-related shift from stimulation to inhibition. The changes observed in LDCL emission suggested that the observed damage to embryogenesis, fertilization and mitotic activity may be related, at least partly, to alterations of the embryo prooxidant state. The present data point to developmental, cytogenetic and biochemical changes related to realistic levels of Al(III), Fe(III) and their mixtures, raising concern as to their environmental, occupational and iatrogenic exposures.
Similar content being viewed by others
References
Berggren D, Fiskesjö G (1987) Aluminum toxicity and speciation in soil liquids—Experiments with Allium cepa L. Environ Toxicol Chem 6:771–779
Bernuzzi V, Desor D, Lehr PR (1989) Developmental alterations in offspring of female rats orally intoxicated by aluminum chloride or lactate during gestation. Teratology 40:21–27
Calabrese A, Collier RS, Nelson, DA, MacInnes JR (1973) The toxicity of heavy metals to embryos of the American oyster Crassostrea virginica. Mar Biol 18:162–166
Chakravarty B, Srivastava S (1992) Toxicity of some heavy metals in vivo and in vitro in Helianthus annuus. Mutat Res 283:287–294
Clark RB (1986) Marine Pollution. Clarendon Press, Oxford
Costantini S, Giordano R, Beccaloni E, Perani C, Grego S (1992) Experimental approach to aluminum phytotoxicity. Microchem J 46:20–29
Dahlgren C (1988) Effects on extra- and intracellularly localized, chemattractant-induced, oxygen radical production in neutrophils following modulation of conditions for ligand-receptor interaction. Inflammation 12:335–341
Domingo JL, Gomez M, Sanchez DJ, Llobet JM, Corbella J (1993) Effect of various dietary constituents on gastrointestinal absorption of aluminum from drinking water and diet. Res Comm Chem Pathol Pharmacol 79:377–380
Evans PH, Yano E, Klinowski J, Peterhans E (1992) Oxidative damage in Alzheimer's dementia, and the potential etiopathogenetic role of aluminosilicates, microglia and micronutrient interactions. In: Emerit I, Chance B (eds) Free Radicals and Aging. Birkhäuser, Basel, pp 178–189
Exley C, Birchall JD (1992) The cellular toxicity of aluminum. J Theor Biol 159:83–98
Fiskesjö G (1983) Nucleolar dissolution induced by aluminium in root cells of Allium. Physiol Plant 59:508–511
Ganrot PO (1986) Metabolism and possible health effects of aluminum. Environ Health Persp 65:363–441
Gilani SH, Chatzinoff M (1981) Aluminum poisoning and chick embryogenesis. Environ Res 24:1–5
Gilbert MR, Harding BL, Hoffman PN, Griffin JW, Price DL, Troncoso JC (1992) Aluminum-induced neurofilamentous changes in cultured rat dorsal root ganglia explants. J Neurosci 12:1763–1771
Gyllenhammar, H (1987) Lucigenin chemiluminescence in the assessment of neutrophil superoxide production. J Immunol Meth 97:209–214
Gomex M, Domingo JL, Llobet JM (1991) Developmental toxicity evaluation of oral aluminum in rats: Influence of citrate. Neurotoxicol Teratol 13:323–328
Halliwell B, Gutteridge JMC (1985) Free Radicals in Biology and Medicine. Clarendon, Oxford
Heinecke JW, Shapiro BM (1989) Respiratory burst oxidase at fertilization. Proc Nat Acad Sci USA 86:1259–1263
His E, Seaman M (1993) A simple, rapid and inexpensive method for monitoring pollutant effects on bivalve embryogenesis and larval development. Society of Ecotoxicology and Environmental Safety, Proc Regional Meeting, Rome, 26
Korkina LG, Suslova TB, Kirov CN, Velichkovskii BT (1984) Mechanisms of cytotoxic effects of aluminosilicates. Pharmakol Toksikol 5:63–68
Korkina LG, Durnev AD, Suslova TB, Cheremisini ZP, Daugel-Dauge NO, Afanas'ev IB (1992) Oxygen radical mediated mutagenic effect of asbestos on human lymphocytes: Suppression by oxygen radical scavengers. Mutat Res 265:245–253
Martin RB (1986) The chemistry of aluminum as related to biology and medicine. Clin Chem 32:1797–1806
McCord JM, Fridovich I (1968) The reduction of cytochrome c by milk xanthine oxidase. J Biol Chem 243:5753–5757
Olsson M, Hogstrand C, Dahlberg A, Berglind S-E (1987) Acid-shock, aluminium, and presence of Sphagnum aurantiacum: Effects on embryological development in the common frog, Rana temporaria, and the moor frog, Rana arvalis. Bull Environ Contam Toxicol 39:37–44
Oteiza PI (1994) A mechanism for the stimulatory effect of aluminum on iron-induced lipid peroxidation. Arch Biochem Biophys 308:374–379
Pagano G, Esposito, A, Giordano GG (1982) Fertilization and larval development in sea urchins following exposure of gametes and embryos to cadmium. Arch Environ Contam Toxicol 11:47–55
Pagano G, Esposito A, Bove P, deAngelis M, Rota A, Giordano GG (1983) The effects of hexavalent and trivalent chromium on fertilization and development in sea urchins. Environ Res 30:442–452
Pagano G, Cipollaro M, Corsale G, Esposito A, Ragucci E, Giordano GG, Trieff NM (1986) The sea urchin: Bioassay for the assessment of damage from environmental contaminants. In: Cairns J, Jr (ed) Community Toxicity Testing. Association for Standard Testing and Materials, Philadelphia, pp 66–92
Pagano G, Corsale G, Dinnel PA, Esposito A, Romaña LA (1989) Use of sea urchin sperm and embryo bioassay in testing the sublethal toxicity of realistic pollutant levels. In: Grandjean E (ed) Carcinogenic, Mutagenic and Teratogenic Marine Pollutants: Impact on Human Health and the Environment. Gulf, Houston pp 153–164
Pagano G, Anselmi B, Dinnel PA, Esposito A, Guida M, Iaccarino M, Melluso G, Pascale M, Trieff NM (1993) Effects on sea urchin fertilization and embryogenesis of water and sediment from two rivers in Campania, Italy, Arch Environ Contam Toxicol 25:20–26
Pézérat H, Zalma R, Guignard J, Jaurand MC (1990) Production of oxygen radicals by the reduction of oxygen arising from the surface activity of mineral fibers. In: Bignon J, Peto J, Saracci R (eds) Non Occupational Exposure to Mineral Fibers. IARC Scientific Publication N. 100, IARC, Lyon, pp 100–111
Stankovic A, Mitrovic DR (1991) Aluminum salts stimulate luminolenhanced chemiluminescence production by human neutrophils. Free Rad Res Comm 14:47–55
Tiniakos G, Williams R (1988) Cirrhotic process, liver cell carcinoma and extrahepatic malignant tumors in idiopathic hemocromatosis. Appl Pathol 6:128–138
Toyokuni S, Sagripanti J-L (1993) Induction of oxidative single- and double-strand breaks in DNA by ferric citrate. Free Rad Biol Med 15:117–123
Trieff NM, Romaña LA, Esposito A, Oral R, Quiniou F, Iaccarino M, Alcock N, Ramanujam VMS, Pagano G (1995) Effluent from bauxite factory induces developmental and reproductive damage in sea urchins. Arch Environ Contam Toxicol 28:173–177
Woelke CE (1972) Development of a receiving water quality bioassay criterion based on the 48-hour Pacific oyster (Crassostrea gigas) embryo. Tech Rep Dept Fish Wash 9:1–93
Woodard-Knight L, Fudge A, Teubner J, Simmer K (1992) Aluminium absorption and antacid therapy in infancy. J Paediatr Child Health 28:257–259
Zar JH (1984) Biostatistical Analysis, 2nd Ed. Prentice-Hall, London
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Pagano, G., His, E., Beiras, R. et al. Cytogenetic, developmental, and biochemical effects of aluminum, iron, and their mixture in sea urchins and mussels. Arch. Environ. Contam. Toxicol. 31, 466–474 (1996). https://doi.org/10.1007/BF00212429
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00212429