Abstract
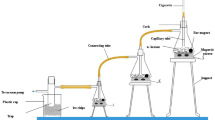
The effect of inhalation of side-stream cigarette smoke on the hepatic microsomal cytochrome P450 was investigated. Rats were placed in a chamber of 0.1 m3 in volume, in which cigarettes were burned at the rate of 1, 3, or 5 cigarettes per h, 8 h/day for 5 days. Cytochrome P450 and NADPH-cytochrome c reductase showed no significant changes; however, cytochrome b5 increased significantly. On the other hand, the activity of aryl hydrocarbon hydroxylase (AHH) decreased in the rats treated with a high concentration of cigarette smoke. In order to study the changes of isoforms of cytochrome P450, western blot analyses were performed. The inductions of three kinds of isoforms, cytochromes P450IA1, IA2, and IIB1, were demonstrated immunochemically. However, there were disagreements between the results of the western blot analyses and the measurements of total cytochrome P450 content and AHH activity.
Similar content being viewed by others
References
Abramson RK, Taylor BA, Tomlin D, Hutton JJ (1977) Genetics of aryl hydrocarbon hydroxylase induction in mice: Response of the lung to cigarette smoke and 3-methylcholanthrene. Biochem Genet 15:723–740
Belisario MA, Borga R, Pecce R, Lorenzo F (1988) Induction of hepatic drug-metabolizing enzymes in rats treated with 1-nitropyrene. Environ Res 45:91–100
Belisario MA, Pecce R, Arena AR, Staiano N (1989) Characterization of the induction of rat hepatic microsomal drug-metabolizing enzymes by 1-nitropyrene metabolites, 1-aminopyrene and N-acetylaminopyrene. Toxicology 57:15–27
Bilimoria MH, Ecobichon DJ (1980) Responses of rodent hepatic, renal and pulmonary aryl hydrocarbon hydroxylase following exposure to cigarette smoke. Toxicology 15:83–89
Emi Y, Omura T (1988). Synthesis of sex specific forms of cytochrome P-450 in rat liver is transiently suppressed by hepatic monooxygenase inducers. J Biochem 104:40–43
Gielen JE, Goujon F, Sele J, Van Cantfort J (1979) Organ specificity of induction of activating and inactivating enzymes of cigarette smoke and cigarette smoke condensate. Arch Toxicol Suppl 2:239–251
Graziano MJ, Dorough HW (1984) Effect of cigarette smoking on hepatic biotransformation in rats. Toxicol Appl Pharmacol 75:229–39
International Agency for Research on Cancer (1986) IARC monographs on the evaluation of the carcinogenic risk of chemicals to humans. Tobacco smoking, Vol. 38. IARC, Lyon
Juchau MR, Namkung MJ, Chao ST (1982) Mono-oxygenase induction in the human placenta. Interrelationships among position specific hydroxylations of 17 beta-estradiol and benzo[a]pyrene. Drug Metab Dispos 10:220–224
Kawamoto T, Hobara T, Nakamura K, Imamura A, Ogino K, Kobayashi H, Iwamoto S, Sakai T (1988) Induction of cytochrome P-450, cytochrome b5, NADPH-cytochrome c reductase and change of cytochrome P-450 isozymes with long-term trichloroethylene treatment. Toxicology 53:239–249
Kawamoto T, Matsuno K, Kayama F, Hirai M, Arashidani K, Yoshikawa M, Kodama Y (1990) Effect of ethylene glycol monomethyl ether and diethylene glycol monomethyl ether on hepatic metabolizing enzymes. Toxicology 62:265–274
Kodama Y, Arashidani K, Yoshikawa M (1983) Simplified analysis of benzo[a]pyrene in airborne particles by high performance liquid chromatography. J Chromatogr 261:103–110
Kushinsky R, Louis CJ (1976) The effect of cigarette smoke on aryl hydrocarbon hydroxylase activity and cytochrome P-450 content in rat liver and lung microsome. Oncology 33:197–200
Kuwahara S, Harada N, Yoshioka H, Miyata T, Omura T (1984) Purification and characterization of four forms of cytochrome P-450 from liver microsomes of phenobarbital-treated and 3-methylcholanthrene treated rats. J Biochem 95:703–714
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriopharge T4. Nature 227:680–685
Lowry OH, Rosenbrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Lu AYH, West SB (1980) Multiplicity of mammalian microsomal cytochrome P-450. Pharmacol Rev 31:277–295
Mussalo-Rauhamaa H, Leppaenen A (1986) Cigarettes as a source of some trace and heavy metals and pesticides in man. Arch Environ Health 41:49–55
Nebert DW, Gelboin HV (1968) Substrate-inducible microsomal aryl hydroxylase in mammalian cell culture. I. Assay and properties of induced enzymes. J Biol Chem 243:6242–6249
Nebert DW, Adenik M, Coon MJ, Eastabrook RW, Gonzalez FJ, Guengerich FP, Gunsalus IC, Johnson EF, Kemper B, Levin W, Phillips IR, Sato R, Waterman MR (1987) The P450 gene superfamily: Recommended nomenclature. DNA 6:1–11
Omura T, Sato R (1964) The carbon monoxide binding pigment of liver microsome. Evidence for its hemoprotein nature. J Biol Chem 239:2370–2385
Raunio H, Vahakangas K, Saarni H, Pelkonen O (1983) Effects of cigarette smoke on rat lung and liver ornithine decarboxylase and aryl hydrocarbon hydroxylase activities and lung benzo[a]pyrene metabolism. Acta Pharmacol Toxicol 52:168–174
Ryan DE, Thomas PE, Korzeniowski D, Levin W (1979) Separation and characterization of highly purified forms of liver microsomal cytochrome P-450 from rats treated with polychlorinated biphenyls, phenobarbital and 3-methylchlanthrene. J Biol Chem 254:1365–1374
Snyder R, Remmer H (1982) Hepatic cytochrome P-450 mono-oxygenase system. In: Schenkman JB, Kupfer D (eds) International encyclopedia of pharmacology and therapeutics. Pergamon Press, Oxford, Chapter 8, pp 227–268
Strobel HW, Dignam JD (1978) Purification and properties of NADPH-cytochrome P-450 reductase. Meth Enzymol 52:89–90
Tanaka I, Ishimatsu S, Higashi T, Katoh T, Akiyama T (1990) A passive tobacco smoke exposure system for laboratory animals. J UOEH 12:37–42
Van der Hoeven TA, Coon MJ (1974) Preparation and properties of partially purified cytochrome P-450 and reduced nicotinamide adenine denucleotide phosphate-cytochrome P-450 reductase from rabbit liver microsomes. J Biol Chem 249:6302–6310
Wynder EL, Hoffman D (1968) Experimental tobacco carcinogenesis. Science 162:862–871
Yoshikawa M, Arashidani K, Kodama Y (1987) Analysis of 3-hydroxybenzo[a]pyrene. Jpn J Hyg 42:268
Yoshioka H, Miyata T, Omura T (1984) Induction of a phenobarbital-inducible form of cytochrome P-450 in rat liver microsomes by 1,1-di(p-chlorophenyl)-2,2-dichloroethylene. J Biochem 95:937–947
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kawamoto, T., Yoshikawa, M., Matsuno, K. et al. Effect of side-stream cigarette smoke on the hepatic cytochrome P450. Arch. Environ. Contam. Toxicol. 25, 255–259 (1993). https://doi.org/10.1007/BF00212138
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00212138