Summary
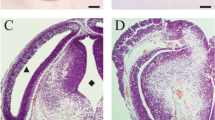
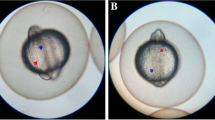
Head-fold stage rat embryos (9.5 days of gestation) were cultured for 48 h in rat serum with or without 0.8 μM 5-azacytidine. Incomplete closure of the cephalic neural tube was observed in 5-azacytidine-treated embryos cultured for 48 h (25-somite stage). Control embryos showed complete fusion of cephalic neural folds at 33 h (16-somite stage) in culture. Drug administration or removal experiments revealed that embryos were sensitive to 5-azacytidine during 6–12 h of culture (three to five somite stages). Electron microscopical studies indicated that the arrangement and fine structure of cephalic neuroepithelial cells were almost the same in control and treated embryos. There was no significant difference in DNA and protein contents between control and treated embryos cultured for 36 h. Immunocytochemical observations using 5-methylcytosine-specific antibody revealed that the staining of neuroepithelial cells in the median part of the transversely sectioned cephalic neural plate, and of mesenchymal cells near the apices of the plate, was suppressed by 5-azacytidine. These results suggest that DNA methylation of these cells plays an important role in closure of the cephalic neural tube.
Similar content being viewed by others
References
Benedict WF, Banerjee A, Gardner A, Jones PA (1977) Induction of morphological transformation in mouse C3H/10T1/2 clone 8 cells and chromosomal damage in hamster A(T1)C1–3 cells by cancer chemotherapeutic agents. Cancer Res 37:2202–2208
Bird A (1978) Use of restriction enzymes to study eukaryotic DNA methylation: II. The symmetry of methylated sites supports semi-conservative copying of the methylation pattern. J Mol Biol 118:49–60
Brown NA, Fabro S (1981) Quantitation of rat embryonic development in vitro: a morphological scoring system. Teratology 24:654–678
Burton KA (1956) A study of the conditions and mechanisms of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J 62:315–323
Constantinides PG, Taylor SM, Jones PA (1978) Phenotypic conversion of cultured mouse embryo cells by aza-pyrimidine nucleosides. Dev Biol 66:57–71
Erlanger BF, Beiser SM (1964) Antibodies specific for ribonucleosides and ribonucleotides and their reaction with DNA. Proc Natl Acad Sci USA 52:68–74
Friedman S (1979) The effect of 5-azacytidine on E. coli DNA methylase. Biochem Biophys Res Commun 89:1328–1333
Gruenbaum Y, Ceder H, Razin A (1982) Substrate and sequence specificity of a eukaryotic DNA methylase. Nature 295:620–622
Holliday R, Pugh E (1975) DNA modification mechanisms and gene activity during development. Science 187:226–232
Hsiao WW, Gattoni-Celli S, Kirshmeire P, Weinstein IB (1984) Effects of 5-azacytidine on methylation and expression of specific DNA sequences in C3H10T/2 cells. Mol Cell Biol 4:634–641
Jones PA, Taylor SM (1980) Cellular differentiation, cytodine analogs and DNA methylation. Cell 20:85–93
Jones PA, Taylor SM (1981) Hemimethylated duplex DNAs prepared from 5-azacytidine-treated cells. Nucl Acids Res 9:2933–2947
Konieczny SF, Emerson CP (1984) 5-Azacytidine induction of stable mesodermal stem cell lineages from 10T1/2 cells: evidence for regulatory genes controlling determination. Cell 38:791–800
Li LH, Olin J, Buskirk HH, Reineke LM (1970a) Cytotoxicity and mode of action of 5-azacytidine on L1210 leukemia. Cancer Res 30:2760–2769
Li LH, Olin J, Fraser TJ, Bhuyan BK (1970b) Phase-specificity of 5-azacytidine against mammalian cells in tissue culture. Cancer Res 30:2770–2769
Lu LW, Randerth K (1979) Effects of 5-azacytidine on transfer RNA methyltransferases. Cancer Res 39:940–949
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the phenol reagent. J Biol Chem 193:265–275
Matsuda M (1990) Fusion of neural folds in the rhombencephalic region of rat embryos. Dev Growth Differ 32:383–388
Matsuda M, Kimura R, Shoji R (1987) Effect of 5-azacytidine on rat embryonic development in vitro. Zool Sci 4:1056
New DAT, Coppola PT, Cockroft DL (1976) Comparison of growth in vitro and in vivo of post-implantation rat embryos. J Embryol Exp Morphol 36:133–144
Reznikoff CA, Brankow DW, Heidelberger C (1973) Establishment and characterization of a cloned line of C3H mouse embryo cells sensitive to postconfluence inhibition of division. Cancer Res 33:3231–3238
Sager R, Kovac P (1982) Pre-adipocyte determination either by insulin or by 5-azacytidine. Proc Natl Acad Sci USA 79:480–484
Santi DV, Garret CE, Barr PJ (1983) On the mechanism of inhibition of DNA-cytosine methyltransferases by cytosine analogs. Cell 33:9–10
Sato M, Azuma M, Hayashi H, Yanagawa H, Yura Y (1987) 5-Azacytidine induction of stable myoepithelial and acinar cells from a human salivary intercalated duct cell clone. Cancer Res 47:4453–4459
Taylor SM, Jones PA (1979) Multiple new phenotypes induced in 10T1/2 and 3T3 cells treated with 5-azacytidine. Cell 17:771–779
Takeuchi IK, Takeuchi YK (1978) Cell death induced by 5-azacytidine in the telencephalic wall of the rat fetus. Annot Zool Jpn 51:10–19
Takeuchi IK, Takeuchi YK (1985) 5-Azacytidine-induced exencephaly in mice. J Anat 140:403–412
Wigler MH (1981) The inheritance of methylation patterns in vertebrates. Cell 24:285–286
Wigler M, Levy D, Perucho M (1981) The somatic replication of DNA methylation. Cell 24:33–40
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Matsuda, M., Yasutomi, M. Inhibition of cephalic neural tube closure by 5-azacytidine in neurulating rat embryos in vitro. Anat Embryol 185, 217–223 (1992). https://doi.org/10.1007/BF00211820
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00211820