Abstract
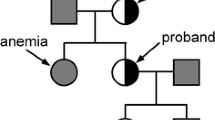
X-linked sideroblastic anemia is a genetic disorder characterized by a hypochromic microcytic anemia of variable intensity with the presence of ring sideroblasts in the bone marrow of the patients. Two different mutations have been reported in the ALAS2 gene in patients with this diseae. We have studied a large kindred with a pyridoxine-sensitive form of X-linked sideroblastic anemia. Sequencing amplified cDNA of the proband revealed a guanine-to-adenine change at nucleotide 871 of the coding sequence (exon 7 of the gene). This results in a glycine to serine substitution that is responsible for a marked decrease in the enzymatic activity of the mutated protein. A polymerase chain reaction assay demonstrated the presence of the same mutation in three affected males and two female carriers in the kindred. The carrier status was excluded in eight females at risk. Early detection of the mutant allele in family members may thus be important for the prevention of anemia in males and of iron overload both in affected males and carrier females.
Similar content being viewed by others
References
Aoki Y, Urata G, Takaku F (1973) Delta-aminolevulinic acid synthetase activity in erythroblasts of patients with primary sideroblastic anemia. Acta Haematol Jpn 36:74–77
Aoki y, Muranaka S, Nakabayashi K, Ueda Y (1979) Deltaaminolevulinic acid synthetase in erythroblasts of patients with pyridoxine-responsive anemia: hypercatabolism caused by the increased susceptibility to the controlling protease. J Clin Invest 64:1196–1203
Astrin KH, Bishop DF (1989) Assignment of human erythroid delta-aminolevulinate synthase (ALAS2) to the X chromosome (abstract). Cytogenet Cell Genet 51:953–954
Bishop DF (1990) Two different genes encode delta-aminolevulinate synthase in humans: nucleotide sequences of cDNAs for the housekeeping and erythroid genes. Nucleic Acids Res 18: 7187–7188
Bishop DF, Henderson AS, Astrin KH (1990) Human deltaaminolevulinate synthase: assignment of the housekeeping gene to 3q21 and the erythroid-specific gene to the X chromosome. Genomics 7:207–214
Bottomley SS (1993) Sideroblastic anemias. In: Lee GR, Bithell TC, Foerster J, Athens JW, Lukens JN (eds) Wintrobe's clinical hematology, vol 1, 9th edn. Lea & Febiger, Philadelphia, pp 852–871
Byrd RB, Cooper T (1961) Hereditary iron-loading anemia with secondary hemochromatosis. Ann Intern Med 55:103–123
Conboy JG, Cox TC, Bottomley SS, Bawden MJ, May BJK (1992) Human erythroid 5-aminolevulinate synthase. Gene structure and species-specific. Differences in alternative RNA splicing. J Biol Chem 267:18753–18758
Cotter PD, Willard HF, Gorski JL, Bishop DF (1992a) Assignment of human erythroid delta-aminolevulinate synthase (ALAS2) to a distal subregion of band Xp11.21 by PCR analysis of somatic cell hybrids containing X; autosome translocations. Genomics 13:211–212
Cotter PD, Baumann M, Bishop DF (1992b) Enzymatic defect in ‘X-linked’ sideroblastic anemia: molecular evidence for erythroid delta-aminolevulinate synthase deficiency. Proc Natl Acad Sci USA 89:4028–4032
Cox TC, Bawden MJ, Alison M, Martin A, May BK (1991) Human erythroid 5-aminolevulinate synthase: promoter analysis and identification of an iron-responsive element in the mRNA. EMBO J 10:1891–1902
Cox TC, Bottomley SS, Wiley JS, Bawden MJ, Matthews CS, May BK (1994) X-linked pyridoxine-responsive sideroblastic anemia due to a thr388-to-ser substitution in erythroid 5-aminolevulinate synthase. N Engl J Med 330:675–679
Ferreira GC, Dailey HA (1993) Expression of mammalian 5-aminolevulinate synthase in Escherichia coli. Overproduction, purification, and characterization. J Biol Chem 268:584–590
Hutman T, Stahl S, Horns E, Mathias U (1989) Direct sequencing of genomic and plasmid DNA using magnetic beads as solid support. Nucleic Acids Res 17:4937–4946
Kan YW, Holland JP, Dozy AM, Varmus HE (1975) Demonstration of non-functional β-globin mRNA in homozygous β ∘thalassemia. Proc Natl Acad Sci USA 72:5140–5144
Peto TEA, Pippard MJ, Weatherall DJ (1983) Iron overload in mild sideroblastic anaemias. Lancet 1:375–378
Schoenhaut DS, Curtis PJ (1986) Nucleotide sequence of mouse 5-aminolevulinic acid synthase cDNA and expression of its gene in hepatic and erythroid tissues. Gene 48:55–63
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Prades, E., Chambon, C., Dailey, T.A. et al. A new mutation of the ALAS2 gene in a large family with X-linked sideroblastic anemia. Hum Genet 95, 424–428 (1995). https://doi.org/10.1007/BF00208968
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00208968