Abstract
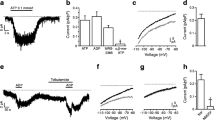
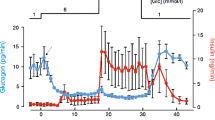
Modulation of the Ca- and voltage-dependent K channel—KCa—by receptors coupled to the G proteins G i /G o and G s has been studied in insulin-secreting cells using the patch clamp technique. In excised outside-out patches somatostatin (somatotropin-releasing inhibitory factor; SRIF) caused concentration-dependent inhibition of the KCa channel, an effect that was prevented by pertussis toxin (PTX). In inside-out patches, exogenous α subunits of either G i or G o -type G proteins also inhibited the KCa channel (IC50 5.9 and 5.7 pM, respectively). These data indicate that SRIF suppresses KCa channel activity via a membrane-delimited pathway that involves the α subunits of PTX-sensitive G proteins G i and/or G o . In outside-out patches, activation of G s either by β-agonists or with cholera toxin (CTX) increased KCa channel activity, consistent with a membrane-delimited stimulatory pathway linking the β-adrenergic receptor to the KCa channel via G s . In outside-out patches, channel inhibition by SRIF suppressed the stimulatory effect of β-agonists but not that of CTX, while in inside-out patches CTX reversed channel inhibition induced by exogenous α i or α o . Taken together these data suggest that KCa channel activity is enhanced by activation of G s and blocked by activated G i and/or G o . Further, KCa channel stimulation by activated G s may be “direct,” while inhibition by G i /G o may involve deactivation of G s . In inside-out patches KCa channel activity was reduced by an activator of protein kinase C (PKC) and enhanced by inhibitors of PKC, indicating that PKC also acts to inhibit the KCa channel via a membrane delimited pathway. In outside-out patches, chelerythrine, a membrane permeant inhibitor of PKC prevented the inhibitory effect of SRIF, and in inside-out patches PKC inhibitors prevented the inhibitory effect of exogenous α i or α o . These data indicate that PKC facilitates the inhibitory effect of the PTX-sensitive G proteins which are activated by coupling to SRIF receptors. To account for these results a mechanism is proposed whereby PKC may be involved in G i /G o -induced deactivation of G s .
Similar content being viewed by others
References
Ahrén, B., Taborsky, Jr., G.J., Porte, Jr., D. 1986. Neuropeptidergic versus cholinergic and adrenergic regulation of islet hormone section. Diabetologia 29:827–836
Bezanilla, F. 1985. A high capacity data recording device based on a digital audio processor and a video cassette recorder. Biophys. J. 47:437–441
Birnbaumer, L., Abramowitz, J., Brown, A.M. 1990. Receptor-effector coupling by G proteins. Biochim, Biophys. Acta 1031:163–224
Bushfield, M., Murphy, G.J., Lavan, B.E., Parker, P.J., Hruby, V.J., Milligan, G., Houslay, M.D. 1990. Hormonal regulation of Gi2 α-subunit phosphorylation in intact hepatocytes. Biochem. J. 268:449–457
Cotroneo, P., Marie, J-C, Rosselin, G. 1988. Characterization of covalently cross-linked somatostatin receptors in hamster beta cell insulinoma. Eur. J. Biochem. 174:219–224
Gilman, A.G. 1987. G proteins: transducers of receptor-generated signals. Annu. Rev. Biochem. 56:615–649
Gollasch, M., Kleuss, C., Hescheler, J., Wittig, B., Schultz, G. 1993. Gi2 and protein kinase C are required for thyrotropin-releasing hormone-induced stimulation of voltage-dependent Ca2+ channels in rat pituitary GH3 cells. Proc. Nat. Acad. Sci. 90:6265–6269
Herbert, J.M., Augereau, J.M., Gleye, J., Maffrand, J.P. 1990. Chelerythrine is a potent and specific inhibitor of protein kinase C. Biochem. Biophys. Res. Commun. 172(3):993–999
Hidaka, H., Inagaki, M., Kawamoto, S., Sasaki, Y. 1984. Isoquinolinesulfonamides, novel and potent inhibitors of cyclic nucleotide dependent protein kinase and protein kinase C. Biochemistry 23:5036–5041
House, C., Kemp, B.E. 1987. Protein kinase C contains a pseudosubstrate prototope in its regulatory domain. Science 238:1726–1728
Jakobs, K.H., Bauer, S., Watanabe, Y. 1985. Modulation of adenylate cyclase of human platelets by phorbol ester. Impairment of the hormone-sensitive inhibitory pathway. Eur. J. Biochem. 151:425–430
Katada, T., Oinuma, M., Ui, M. 1986. Two guanine nucleotide-binding proteins in rat brain serving as the specific substrate of islet-activating protein, pertussis toxin. J. Biol. Chem. 261:8182–8191
Katada, T., Ui, M. 1982. Direct modification of the membrane adenylate cyclase system by islet-activating protein due to ADP-ribosylation of a membrane protein. Proc. Nat. Acad. Sci. 79:3129–3133
Knöpfel, T., Vranesic, I., Gähwiler, B.H., Brown, D.A. 1990. Muscarinic and β-adrenergic depression of the slow Ca2+-activated potassium conductance in hippocampal CA3 pyramidal cells is not mediated by a reduction of depolarization-induced cytosolic Ca2+ transients. Proc. Natl. Acad. Sci. 87:4083–4087
Kukuljan, M., Goncalves, A.A., Atwater, I. 1991. Charybdotoxin-sensitive KCa channel is not involved in glucose-induced electrical activity in pancreatic β-cells. J. Membrane Biol. 119:187–195
Madison, D.V., Nicoll, R.A. 1986. Cyclic adenosine 3′,5′-monophosphate mediates β-receptor actions of noradrenaline in rat hippocampal pyramidal cells. J. Physiol. 372:245–259
Maletti, M., Andersson, M., Marie, J-C., Rosselin, G., Mutt, V. 1992. Solubilization and partial purification of somatostatin-28 preferring receptors from hamster pancreatic β cells. J. Biol. Chem. 267(22): 15620–15625
Malenka, R.C., Madison, D.V., Andrade, R., Nicoll, R.A. 1986. Phorbol esters mimic some cholinergic actions in hippocampal pyramidal neurons. J. Neurosci. 6(2):475–480
Nishino, H., Kitagawa, K., Iwashima, A., Ito, M., Tanaka, T., Hidaka, H. 1986. N-(6-Phenylhexyl)-5-chloro-1-naphthalenesulfonamide is one of a new class of activators for Ca2+ activated, phospholipid-dependent protein kinase. Biochim. Biophys. Acta 889:236–239
Okamoto, T., Murayama, Y., Hayashi, Y., Inagaki, M., Ogata, E., & Nishimoto, I. 1991. Identification of a G s activator region of the β 2-adrenergic receptor that is autoregulated via protein kinase A-dependent phosphorylation. Cell 67:723–730
Ribalet, B., Beigelman, P.M. 1981. Effects of divalent cations on β-cell electrical activity. Am. J. Physiol. 241:C59-C67
Ribalet, B., S. Ciani. 1994. Characterization of the G protein coupling of a glucagon receptor to the KATP channel in insulin secreting cells. J. Membrane Biol. 142:395–408
Ribalet, B., Ciani, S., Eddlestone, G.T. 1989. Modulation of ATP-sensitive K channels in RINrn5F cells by phosphorylation and G proteins. Biophys. J. 55:587a
Ribalet, B., Ciani, S., Hales, T.G., Eddlestone, G.T. 1991. Role of G proteins in K(ATP) channel modulation by the neuropeptide somatostatin. Biomed. Res. 12(2):37–40
Ribalet, B., Eddlestone, G.T. 1995. Characterization of the G protein coupling of a SRIF receptor to the KATP channel in insulin secreting cells. J. Physiol. 485.1:73–86
Ribalet, B., Eddlestone, G.T., Ciani, S. 1988. Metabolic regulation of the K(ATP) and a Maxi-K(V) channel in the insulin secreting RINm5F cell. J. Gen. Physiol. 92:219–237
Scornik, F.S., Codina, J., Garcia-Calvo, M., Kraus, M-J., Birnbaumer, L., Garcia, M.L., Toro, L. 1994. G s protein activation of a purified KCa channel reconstituted in lipid bilayers. Biophys. J. 66(2): 342a
Sibley, D.R., Lefkowitz, R.J. 1985. Molecular mechanisms of receptor desensitization using the β-adrenergic receptor-coupled adenylate cyclase system as a model. Nature 317:124–129
Sternweis, P.C., Smrcka, A.V. 1992. Regulation of phospholipase C by G proteins. Trends Biochem. Sci. 17:502–506
Toro, L., Ramos-Franco, J., Stefani, E. 1990. GTP-dependent regula7tion of myometrial KCa channels incorporated into lipid bilayers. J. Gen. Physiol. 96:373–394
White, R.E., Schonbrunn, A.E., Armstrong, D.L. 1991. Somatostatin stimulates Ca2+-activated K+ channels through protein dephosphorylation. Nature 351:570–573
Yatani, A., Codina, J., Brown, A.M., Birnbaumer, L. 1987. Direct activation of mammalian atrial muscarinic potassium channels by GTP regulatory protein G k Science 235:207–211
Author information
Authors and Affiliations
Additional information
The authors would like to thank Dr. S. Ciani for many helpful discussions, Dr. A.E. Boyd III for supplying the HIT cells, Drs. J. Codina and L. Birnbaumer for supplying the alpha subunits of the G proteins G i and G o , and Mrs. Satoko Hagiwara for preparing and maintaining the cell cultures.
This work was supported by grant DCB-8919368 from the National Science Foundation and a research grant (W-P 880513) from the American Diabetes Association to B.R., and by grant RO1-DK39652 from the National Institutes of Health to G.T.E.
Rights and permissions
About this article
Cite this article
Ribalet, B., Eddlestone, G.T. Characterization of the G protein coupling of SRIF and β-adrenergic receptors to the maxi KCa channel in insulin-secreting cells. J. Membarin Biol. 148, 111–125 (1995). https://doi.org/10.1007/BF00207268
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00207268