Summary
The synchronizing effect of ethinylestradiol (4 μg/g b.w.) on neurons of the arcuate nucleus 700–950 μm caudal to the posterior edge of the optic chiasma was studied by karyometry in 6-week-old albino mice during proestrus.
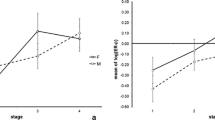
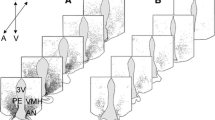
The caudal portion of the arcuate nucleus was identified as the most estrogen-sensitive subdivision; all neurons showed an increase in their nuclear area (mean transect, profile area of the nucleus) 1 h following administration of ethinylestradiol. This hypothalamic region was selected for the subsequent electron-microscopic cytometric study to analyze functional interrelationships among neurons, ependymal cells and glial cells. Six and 12 days after ovariectomy no significant change in the nuclear area of neurons and ependymal cells was found 850–950 μm behind the posterior slope of the optic chiasma, but the neurons exhibited a decrease in the number of polyribosomes, the volume fraction (VVmi) and the surface density of the inner membrane of mitochondria (SVmi). A similar decrease in VVmi and SVmi was measured in the apical part of ependymal cells and in the pericapillary profiles of ependymal and glial cells, which was accompanied by a reduction in the surface density of ependymal processes extending into the ventricular lumen. In addition, no change of VVmi and SVmi was seen in the basal subnuclear part of ependymal cells.
This bipolar functional reaction of ependymal cells after ovariectomy is discussed as an indicator of ependymal control of neuronal activity by sequestering biologically active agents, e.g., transmitters of neurohormones, in their apical and basal extensions facing the ventricular surface or the pericapillary space.
Similar content being viewed by others
References
Anand Kumar TC, Thomas GH (1968) Metabolites of 3H-estradiol-17 beta in the cerebrospinal fluid of the Rhesus monkey. Nature 219:628–629
Araki S, Ferin E, Zimmerman E, Van de Wiele RL (1975) Ovarian modulation of immunoreactive gonadotropin-releasing hormone (Gn-RH) in the rat brain: Evidence for a differential effect on the anterior and mid-hypothalamus. Endocrinology 96:644–650
Baulieu EE (1978) Wirkungsmechanismus der Östrogene. Physiologische Aspekte der hormonellen “Rezeptivität”. Die Anti-Östrogene. Klin Wschr 56:683–695
Benninghoff A (1950) Funktionelle Kernschwellung und Kernschrumpfung. Anat Nachr 1:50–52
Brightman MW, Reese TS (1969) Functions between intimately opposed cell membranes in the vertebrate brain. J Cell Biol 40:648–677
Bueno J, Pfaff DW (1976) Single unit recording in hypothalamus and preoptic area of estrogen-treated and untreated ovariectomized female rats. Brain Res 101:67–78
Bugnon C, Bloch B, Lenys D, Gouget A, Fellmann D (1979) Comparative study of the neuronal populations containing β-endorphin, corticotropin and dopamine in the arcuate nucleus of the rat hypothalamus. Neurosci Lett 14:43–48
Dahlström A, Fuxe K (1964) Evidence for the existence of monoamine-containing neurons in the central nervous system. I. Demonstration of monoamines in the cell bodies of brain stem neurons. Acta Physiol Scand 62 suppl 232:1–55
Demarest KT, Johnston CA, Moore KE (1981) Biochemical indices of catecholaminergic neuronal activity in the median eminence during the estrous cycle of the rat. Neuroendocrinology 32:24–27
Eulig HG, Mond W (1952) Der Einfluß der Fixierung auf das Kernvolumen. Z wiss Mikr 61:201–209
Fischmeister HF (1967) Apparative Hilfsmittel in der Stereologie. In: Weibel ER, Ellias H (eds) Quantitative Methoden der Morphologie. Springer, Berlin Heidelberg New York, pp 221–249
Foreman MM, Wickersham EV, Anthony A (1977) Cytophotometric analysis of hypothalamic RNA fluctuation during the rat estrous cycle. Brain Res 119:471–475
Fuxe K, Hökfelt T, Agnati L, Löfström A, Everitt BJ, Johansson O, Jonsson G, Wuttke W, Goldstein M (1976) Role of monoamines in the control of gonadotropin secretion. In: Anand Kumar TC (ed) Neuroendocrine regulation of fertility. Karger, Basel, pp 124–140
Grant LD, Heritage AS, Stumpf WE (1977) Localization of 3H-estradiol uptake sites in relation to CNS catecholamine pathways by combined fluorescence microscopy/autoradiography techniques. Acta Pharmacol Toxicol 41:44–45
Grünthal E (1930) Vergleichende anatomische und entwicklungsgeschichtliche Untersuchungen über die Zentren des Hypothalamus der Säuger und der Menschen. Arch Psychiat Nervenkr 90:216–267
Gudelsky GA, Annunziato L, Moore KE (1977) Increase in dopamine content of the rat median eminence after long-term ovariectomy and its reversal by estrogen replacement. Endocrinology 101:1894–1897
Güldner FH, Wolff JR (1973) Neurono-glial synaptoid contacts in the median eminence of the rat: Ultrastructure, staining properties and distribution on tanycytes. Brain Res 61:217–234
Hertl M (1953) Brunstzeitige Kernschwellung im Tuber cinereum der weißen Maus. Morph Jb 92:75–94
Hösli L, Hösli E (1978) Action and uptake of neurotransmitters in CNS tissue culture. Rev Physiol Biochem Pharmacol 81:135–188
Hoffman GE, Knigge KM, Moynihan JA, Melnyk V, Arimura A (1978) Neuronal fields containing luteinizing hormone releasing hormone (LHRH) in mouse brain. Neuroscience 3:219–231
Jennes L, Stumpf WE (1980) LHRH-systems in the brain of the golden hamster. Cell Tissue Res 209:239–256
Kawakami M, Kubo K (1971) Neuro-correlate of limbic-hypothalamo-pituitary-gonadal axis in the rat: Change in limbic-hypothalamic unit activity induced by vaginal and electrical stimulation. Neuroendocrinology 7:65–74
King JC, Williams TH, Gerall AA (1974) Transformations of hypothalamic arcuate neurons. I. Changes associated with stages of the estrous cycle. Cell Tissue Res 153:497–515
Knigge KM (1975) Opening remarks. In: Knigge KM, Scott DE, Kobayashi H, Ishii S (eds) Brainendocrine interaction II. The ventricular system in neuroendocrine mechanisms. Karger, Basel
Knowles F, Anand Kumar TC (1969) Structural changes, related to reproduction, in the hypothalamus and in the pars tuberalis of the rhesus monkey. Phil Trans B 256:357–375
Knowles F, Vollrath L (1965) Synaptic contacts between neurosecretory fibres and pituicytes in the pituitary of the eel. Nature 206:1168–1169
Knowles F, Vollrath L (1966) A functional relationship between neurosecretory fibres and pituicytes in the eel. Nature 208:1343
Kobayashi H (1975) Absorption of cerebrospinal fluid by ependymal cells of the median eminence. In: Knigge KM, Scott DE, Kobayashi H, Ishii S (eds) Brain-endocrine interaction II. The ventricular system in neuroendocrine mechanisms. Karger, Basel, pp 109–122
Krisch B (1980) Immunocytochemistry of neuroendocrine systems Progr Histochem Cytochem 13
Krisch B, Leonhardt H, Buchheim W (1978) The functional and structural border of the neurohemal region of the median eminence. Cell Tissue Res 192:327–339
Kuffler SW, Nicholls JG (1966) The physiology of neuroglial cells. Rev Physiol Biochem Pharmacol 57:1–90
Litteria M (1973) In vivo alterations in the incorporation of [3H] lysine into the medial preoptic nucleus and specific hypothalamic nuclei during the estrous cycle of the rat. Brain Res 55:234–237
Luine VN, Khylchevskaya RI, McEwen BS (1975) Effect of gonadal hormones on enzyme activities in brain and pituitary of male and female rats. Brain Res 86:283–292
McEwen BS, Krey LC, Luine VN (1978) Steroid hormone action in the neuroendocrine system: When is the genome involved? In: Reichlin S, Baldessarini RJ, Martin JB (eds) The hypothalamus. Raven Press, New York, pp 255–268
Mueller GC, Herranen AM, Werrell KF (1958) Studies on the mechanisms of action of estrogens. Recent Progr Hormone Res 14:95–139
Oksche A (1978a) Evolution, differentiation and organization of hypothalamic systems controlling reproduction. In: Scott DE, Kozlowski GP, Weindl A (eds) Brain-endocrine interaction III. Neural hormones and reproduction. 3rd Int Symp, Würzburg 1977, Karger, Basel pp 1–15
Oksche A (1978b) Pattern of neuroendocrine cell complexes (subunits) in hypothalamic nuclei: neurobiological and phylogenetic concepts. In: Bargmann W, Oksche A, Polenov A, Scharrer B (eds) Neurosecretion and neuroendocrine activity. Evolution, structure and function, Springer, Berlin Heidelberg New York, pp 64–71
Oksche A, Zimmermann P, Oehmke HJ (1972) Morphometric studies of tubero-eminential systems controlling reproductive functions. In: Knigge KM, Scott DE, Weindl A (eds) Brain-endocrine interaction. Median eminence: Structure and function, Karger, Berlin, pp 142–153
Oksche A, Oehmke HJ, Hartwig HG (1974) A concept of neuroendocrine cell complexes. In: Knowles F, Vollrath L (eds) Neurosecretion — The final neuroendocrine pathway, Springer, Berlin, pp 154–164
Pfaff DW (1980) Estrogens and brain function, Springer, New York Heidelberg Berlin
Sarkar DK, Chiappa SA, Fink G (1976) Gonadotropin-releasing hormone surge in pro-estrous rats. Nature (Lond.) 264:401–403
Schmidt M, Zimmermann P (1978) Reactivity patterns of nerve cell-glia-complexes in mice during pentylenetetrazole-induced seizures. Cytometric-cytophotometric studies. Acta Neuropathol (Berl.) 43:243–250
Schneider A (1976) Reaktionsmuster von Neuronenkomplexen im Nucleus arcuatus der Albinomaus. Karyometrische und cytophotometrische Studien. Med Diss, Justus Liebig-Univ. Giessen
Schrier BK, Thompson EJ (1974) On the role of glial cells in the mammalian nervous system. J Biol Chem 249:1769–1780
Somjen GG (1979) Extracellular potassium in the mammalian central nervous system. Ann Rev Physiol 41:159–177
Stumpf WE, Sar M (1975) Hormone-architecture of the mouse brain with 3H-estradiol. In: Stumpf WE, Grant LD (eds) Anatomical neuroendocrinology, Karger, Basel pp 82–103
Vértes M, Göcze P, Varga P, Kovács S (1977) Studies on hypothalamic estradiol binding during estrous cycle and after ovariectomy. Endocrinol Exp 11:227–234
Weibel ER (1969) Stereological principles for morphometry in electron microscopic cytology. Int Rev Cytol 26:235–302
Weibel ER (1973) Stereological techniques for electron microscopic morphometry. In: Hayat MA (ed) Principles and techniques of electron microscopy Vol. 3. Van Nostrand-Reinhold, Amsterdam, pp 237–296
Weibel ER (1979) Stereological methods, Vol. I. Practical methods for biological morphometry. Academic Press, London
Wittkowski W, Scheuer A (1974) Functional changes of the neuronal and glial elements at the surface of the external layer of the median eminence. Z Anat Entwickl-Gesch 143:255–262
Yagi K (1973) Changes in firing rates of single preoptic and hypothalamic units following an intravenous administration of estrogen in the castrated female rat. Brain Res 53:343–352
Zambrano D, de Robertis D (1968) The effect of castration upon the ultrastructure of the rat hypothalamus. II. Arcuate nucleus and outer zone of the median eminence. Z Zellforsch 87:409–421
Zimmermann P (1967) Methodische Modifikationen und eine neue Technik zur Darstellung des neurosekretorischen Apparates und der Neuroglia bei Wirbellosen (Lumbricus terrestris L.) Z wiss Mikr 68:154–162
Zimmermann P (1974) Cytometrische und mikrospektrographische Studien über Wechselbeziehungen zwischen Endothel-, Glia- und Ganglienzellen. Habilitationsschrift, Justus Liebig-Universität, Giessen
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Zimmermann, P. Estrogen-dependent changes in the functional interrelationships among neurons, ependymal cells and glial cells of the arcuate nucleus. Cell Tissue Res. 227, 113–128 (1982). https://doi.org/10.1007/BF00206335
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00206335