Abstract
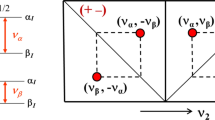
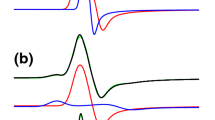
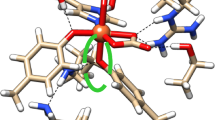
The perturbed angular correlation (PAC) technique has been applied to study the electric quadrupole interaction of 181Hf nuclei at the binding sites of ovotransferrin (OTF) molecules. Two specific electric field gradients were observed. Their relative intensities depend on the pH value and the temperature of the samples, whereas the electric quadrupole interaction parameters themselves remain unaffected. In order to compare the binding sites in OTF, experiments with N- and C-terminal half-molecules were performed. Both specific configurations are observed at the N-terminal and at the C-terminal binding site with similar quadrupole parameters as for the intact protein. Remarkably, the stability of the hafnium binding to the C-terminal fragment appears to be reduced as compared with the N-terminal half and the intact protein.
Similar content being viewed by others
References
Aisen P. 1980 In: Jacobs A, Worwood M, eds. Iron in Biochemistry and Medicine II: The Transferrins. London: Academic Press; 87–129.
Aisen P, Harris DC. 1989 Physical biochemistry of the transferrins. In: Loehr TM, Gray HB, Lever ABP, eds. Iron Carriers and Iron Proteins. Weinheim: VCH Publishers; 239–371.
Anderson BF, Baker HM, Norris GE, Rice DE, Baker EN. 1989 Structure of human lactoferrin: Crystallographic structure analysis and refinement at 2.8 Å resolution. J Mol Biol 209, 711–734.
Anderson BF, Baker HM, Norris GE, Rumball SV, Baker EN. 1990 Apolactoferrin structure demonstrates ligand-induced conformational changes in transferrins. Nature 344, 784–787.
Bailey S, Evans RW, Garrat RC, et al. 1988 Molecular structure of serum transferrin at 3.3 Å resolution. Biochemistry 27, 5804–5812.
Baker EN, Anderson BF, Baker HM, et al. 1991 Structure, function and flexibility of human lactoferrin. Int J Biol Macromol 13, 122–129.
Boyer P, Baudry A. 1984 Perturbed angular correlation of gamma rays. In: Matsuura T, ed. Studies in Physical and Theoretical Chemistry 31: Hot Chemistry. Amsterdam: Elsevier.
Brock J. 1985 Transferrins. In: Harrison PM, ed. Topics in Molecular and Structural Biology, Vol. 7. Weinheim: VCH Publishers; 183–262.
Chasteen ND, Williams J. 1981 The influence of pH on the equilibrium distribution of iron between the metal-sites of human transferrin. Biochem J 193, 717–727.
van Eijk HG, van Noort WL, Kroos MJ, van der Heul C. 1978 Analysis of the iron binding sites of transferrin by isoelectric focussing. J Clin Chem Clin Biochem 16. 475–478.
Frauenfelder H, Steffen RM. 1965 Angular correlations. In: Siegbahn K. ed. Alpha, Beta- and Gamma-Ray Spectroscopy, Vol. 2. Amsterdam: North Holland: 997–1198.
Heidinger R, Thies W-G, Appel H, Then GM. 1987 High resolution 181Hf-TDPAC spectroscopy using fast BaF2 detectors. Hyp Int 35, 1007–1010.
Hutchens TW, Yip TY. 1991 Metal ligand-induced alterations in the surface of lactoferrin and transferrin probed by interaction with immobilized copper (II) ions. J Chromatogr 536, 1–15.
de Jong G, van Dijk JP, van Eijk HG. 1990 The biology of transferrin. Clin Chim Acta 190, 1–46.
Metz-Boutigue M-H, Jolles J, Mazurier J, et al. 1984 Human lactoferrin: amino acid sequence and structural comparison with other transferrins. Eur J Biochem. 145, 659–676.
Oe H, Doi E. Hirose M. 1988 Amino-terminal and carboxyl-terminal half-molecules of ovotransferrin: preparation by a novel procedure and their interactions. J Biochem 103, 1066–1072.
Schwab FJ, Appel H, Neu M, Thies W-G. 1992 Influence of protein dynamics on the metal-sites of ovotransferrin. Eur Biophys J 21, 147–154.
Shinar J, Davidov D, Shaltiel D. 1984 Proton NMR study of diffusion in continuous, nonstoichiometric metal-hydrogen systems. Phys Rev B30, 6331–6336.
Taniguchi T, Ichimura K, Kawashima S, et al. 1990 Binding of Cu(II), Tb(II) and Fe(III) to chicken ovotransferrin. Eur Biophys J 18, 1–8.
Taylor DM, Thies W-G, Appel H, Neu-Müller M, Schwab F, Weber HW. 1988 Spectroscopic studies of hafnium-transferrin-complexes: differences between the two binding sites. In: Hamer DH, Winge DR. eds. Metal Ion Homeostasis: Molecular Biology and Chemistry. Vol. 98. New York: AR Liss: 179–188.
Then GM, Appel H, Raudies JH, Thies W-G, Duffield J, Taylor DM. 1983 The binding of hafnium to nitrilotriacetate. Hyp Int 16, 889–892.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Schwab, F.J., Appel, H., Mason, A.B. et al. Perturbed angular correlation studies of the metal-binding sites in ovotransferrin and its C- and N-terminal halves. Biometals 6, 193–201 (1993). https://doi.org/10.1007/BF00205859
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00205859