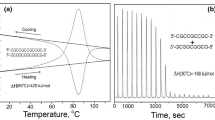
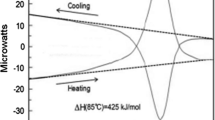
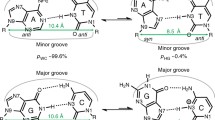
Abstract
We calculate thermal fluctuational base pair opening probability and the drug binding constant of a daunomycin-bound Poly d(CGTA) · Poly d(TACG) at temperatures from room temperature to its melting temperature. For comparison we also carry out a calculation on a drug-free DNA with the same sequence. Our calculations are carried out by means of a statistical approach using microscopic structures and established force fields and with cooperative effects incorporated into the algorithm. Both hydrogen bond disruption probabilities and drug unstacking probability are determined self-consistently. These probabilities are then used to determine temperature dependent base pair opening probabilities and the drug binding constant. The calculated base pair opening probabilities and drug binding constant are found to be in fair agreement with experiments carried out at room temperature. Our calculation shows cooperative base pair disruption and drug dissociation at certain critical temperatures close to the observed melting temperatures for similar helices. We find that the temperature dependence of the drug binding constant fits well to the van't Hoff relation, in agreement with observations. Our calculation indicates the occurrence of a premelting transition in the drug-bound DNA helix. Some comments are made about this premelting transition.
Similar content being viewed by others
References
Blake RD, Delcourt SG (1990) Electrostatic forces at helix-coil boundaries in DNA. Biopolymers 29: 393–405
Califano S (1976) Vibrational states. John Wiley & Sons, New York
Chaires JB (1983) Equilibrium studies on the interaction of daunomycin with deoxypolynucleotides. Biochemistry 22: 4204–4211
Chaires JB (1985) Thermodynamics of the daunomycin-DNA interaction: Ionic strength dependence of the enthalpy and entropy. Biopolymers 24: 403–419
Chaires JB, Herrera JE (1990) Preferential binding of daunomycin to 5′212–1CG and 212–2GC sequences revealed by footprinting titration experiments. Biochemistry 29: 6145–6153
Chaires JB, Dattagupta N, Crothers DM (1982) Studies on interaction of anthracycline antibiotics and deoxyribonucleic acid: Equilibrium binding studies on interaction of daunomycin with deoxyribonucleic acid. Biochemistry 21: 3933–3940
Chandrasekaran R, Arnott S (1989) The structures of DNA and RNA helices in oriented fibers. In: Landolt-Bornstein Numerical Data and Functional Relationships in Science and Technology, Vol. VII/1b. Saenger w (ed) Springer, Berlin Heidelberg New York, pp 31–170
Chen YZ, Prohofsky EW (1992) The role of a minor groove spine of hydration in stabilizing Poly(dA) · Poly(dT) against fluctuational interbase H-bond disruption in the premelting temperature regime. Nucl Acids Res 20: 415–419
Chen YZ, Prohofsky EW (1993a) Differences in melting behavior between homopolymers and copolymers of DNA: Role of nonbonded forces for GC and the role of the hydration spine and premelting transition for AT. Biopolymers 33: 797–812
Chen YZ, Prohofsky EW (1993b) Theoretical study of the effect of salt and the role of strained hydrogen bonds on the thermal stability of DNA polymers. Phys Rev E48: 3099–3106
Chen YZ, Prohofsky EW (1994a) Premelting base pair opening probability and drug binding constant of a daunomycin-Poly d(GCAT) · Poly d(ATGC). Biophys J 66: 820–826
Chen YZ, Prohofsky EW (1994b) Near-neighbor effects in cooperative modified self-consistent approximation melting in DNA. Phys Rev E49: 873–881
Chen YZ, Prohofsky EW (1994c) First or second-order transition in the melting of repeat sequence DNA. Biophys J 66: 202–206
Chen KX, Gresh N, Pullman B (1985) A theoretical investigation on the sequence selective binding of daunomycin to doublestranded polynucleotides. J Biomol Struct Dym 3: 445–466
Chen YZ, Zhuang W, Prohofsky EW (1991) Premelting thermal fluctuational interbase hydrogen-bond disrupted states of a B-DNA guanine-cytosine base pair: Significance for amino and imino proton exchange. Biopolymers 31: 1273–1281
Cieplak P, Rao SN, Grootenhus PDJ, Kollman PA (1990) Free energy calculation on base specificity of drug-DNA interactions: Application to daunomycin and acridine intercalation into DNA. Biopolymers 29: 717–727
Crothers DM (1971) Statistical thermodynamics of nucleic acid melting transitions with coupled binding equilibria. Biopolymers 10: 2147–2160
DiMarco A, Arcamone F, Zunino F (1975) In: Corcoran JW, Hahn FE (eds) Antibiotics, Mechanism of Action of Antimicrobial and Antitumor Agents. Springer, Berlin, pp 101–128
Frederick CA, Williams LD, Ughetto G, ven der Marel GA, van Boom JH, Rich A, Wang AHJ (1990) Structural comparison of anticancer drug-DNA complexes: Adriamycin and daunomycin. Biochemistry 29: 2538–2549
Gueron M, Kochoyan M, Leroy JL (1987) A single mode of DNA base-pair opening drives imino proton exchange. Nature 328: 89–92
Lu KC, Prohofsky EW, Van Zandt LL (1977) Vibrational modes of A-DNA, B-DNA and A-RNA backbones: An application of a Green-function refinement procedure. Biopolymers 16: 2491–2506
McGhee JD (1976) Theoretical calculations of the helix-coil transition of DNA in the presence of large, cooperative binding ligands. Biopolymers 15: 1345–1375
Mei WN, Kohli M, Prohofsky EW, Van Zandt LL (1981) Acoustic modes and nonbonded interactions of the double helix. Biopolymers 20: 833–852
Miller KJ (1979) Interactions of molecules with nucleic acids. I. An algorithm to generate nucleic acid structures with an application to B-DNA structure and a counterclockwise helix. Biopolymers 18: 959–980
Misra VK, Sharp KA, Friedman RA, Honig B (1994) Salt effects on ligand-DNA binding. Minor groove binding anibiotics. J Mol Biol 238: 245–263
Newlin DD, Miller KJ, Pilch DF (1984) Interactions of molecular with nucleic acids. VII. Intercalation and T · A specificity of daunomycin in DNA. Biopolymers 23: 139–158
Nunn CM, Meervelt LV, Zhang S, Moore MH, Kennard O (1991) The crystal structures of d(TGTACA) and d(TGATCA) complexed with daunomycin. J Mol Biol 222: 167–177
Phillips DR, Dimarco A, Zunino F (1978) The interaction of daunomycin with polydeoxynucleotides. Eur J Biochem 85: 487–492
Prabhu VV, Young L, Prohofsky EW, Edwards GS (1989) Hydrogen-bond melting in B-DNA copolymers in a mean field selfconsistent phonon approach. Phys Rev B39: 5436–5443
Prohofsky EW (1985) Motional dynamics of the DNA double helix. In: Biomol. Stereodynamics IV. Proc. 4th Conversation in the Discipline Biomol. Stereodynamics. Samar RH, Samar MH (ed) Adenine press, pp 21–46
Quigley GJ, Wang AH-J, Ughetto G, van der Marel G, van Boom JH, Rich A (1980) Molecular structure of an anticancer drug-DNA complex: Daunomycin plus d(CpGpTpApCpG). Proc Natl Acad Sci USA 77: 7204–7208
Remeta DP, Mudd CP, Berger RL, Breslauer KJ (1993) Thermodynamic characterization of daunomycin-DNA interactions: Comparison of complete binding profiles for a series of DNA host duplexes. Biochemistry 32: 5064–5073
Tsuboi M, Takahashi S, Harada I (1973) Infrared and Raman spectra of nucleic acids — vibrations in the base residues. In: Physico-Chemical Properties of Nucleic Acids. Vol. 2, Duchesne J (ed) Academic Press, New York, pp 91–145
Wang AH-J, Ughetto G, Quigley GJ, Rich A (1987) Interactions between an anthracycline antibiotic and DNA: Molecular structure of daunomycin complexed to d(CpGpTpApCpG) at 1.2-Å resolution. Biochemistry 26: 1152–1163
Weiner SJ, Kollman PA, Nguyen DT, Case DA (1986) An all force field for simulations of proteins and nucleic acids. J Comp Chem 7: 230–252
Wells RD, Larson JE, Grant RC, Shortle BE, Cantor CR (1970) Physiochemical studies of polydeoxyribonucleotides containing defined repeating nucleotide sequences. J Mol Biol 54: 465–497
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Chen, Y.Z., Prohofsky, E.W. Melting profile and temperature dependent binding constant of an anticancer drug daunomycin-DNA complex. Eur Biophys J 24, 203–212 (1996). https://doi.org/10.1007/BF00205101
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00205101