Abstract
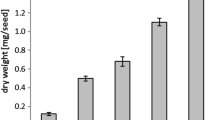
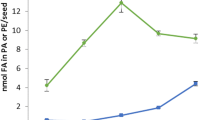
The activity of 1-acylglycerophosphocholine (1-acyl-GPC) O-acyltransferase (EC 2.3.1.23) varied during maturation of safflower (Carthamus tinctorius L.) seeds, and activity per seed was highest in the middle period of seed development when triacylglycerol (TAG) is most rapidly synthesized. The specific activity of acyl transfer in a 20000·g particulate preparation exceeded 500nmol·min-1·(mg protein)-1 and was higher than those of any other enzymes involved in TAG synthesis (K. Ichihara et al., 1993, Plant Cell Physiol. 34, 557–566). This suggested the presence of a large flux of acyl-CoA to phosphatidylcholine in the cell. The reaction was specific to C16 and C18 acyl-CoAs with a double bond at position 9. Lauroyl- and erucoyl-CoA were completely ineffective, while ricinoleoyl- and elaidoyl-CoA were utilized efficiently. The relative order of specificity for native acyl-CoA species was linoleoyl > oleoyl ≫ stearoyl = palmitoyl. When acyl-CoA mixtures were presented, preference for the unsaturated species rather than the saturated species was even more apparent. The enzyme preferentially utilized 1-C16-acyl- and 1-C18-acyl-GPC molecular species, and 1-palmitoyl-, 1-stearoyl-, 1-oleoyl-and 1-linoleoyl-GPC equally served as acyl acceptor. No activity was detected with 1-octanoyl-GPC, and 1-erucoyl-GPC produced little effect. The effectiveness of 1-alkyl-GPC was comparable to that of 1-acyl-GPC. It was thus concluded that the enzyme recognizes the chain lengths of the acyl donor and acceptor, and the double bond at position 9 of the acyl donor.
Similar content being viewed by others
Abbreviations
- DAG:
-
diacylglycerol
- DTNB:
-
5,5′-dithiobis(2-nitrobenzoic acid)
- GP:
-
sn-glycerol 3-phosphate
- GPC:
-
sn-glycero-3-phosphocholine
- GPE:
-
sn-glycero-3-phosphoethanolamine
- GPI:
-
sn-glycero-3-phosphoinositol
- PC:
-
phosphatidylcholine
- TAG:
-
triacylglycerol
References
Bafor, M., Smith, M.A., Jonsson, L., Stobart, K., Stymne, S. (1991) Ricinoleic acid biosynthesis and triacylglycerol assembly in microsomal preparations from developing castor-bean (Ricinus communis) endosperm. Biochem. J. 280, 507–514
Bafor, M., Smith, M.A., Jonsson, L., Stobart, K., Stymne, S. (1993) Biosynthesis of vernoleate (cis-12-epoxyoctadecacis-9-enoate) in microsomal preparations from developing endosperm of Euphorbia lagascae. Arch. Biochem. Biophys. 303, 145–151
Bartels, C.T., van Deenen, L.L.M. (1966) The conversion of lysophosphoglycerides by homogenates of spinach leaves. Biochim. Biophys. Acta 125, 395–397
Battey, J.F., Ohlrogge, J.B. (1989) A comparison of the metabolic fate of fatty acids of different chain lengths in developing oilseeds. Plant Physiol. 90, 835–840
Browse, J., Somerville, C. (1991) Glycerolipid synthesis: biochemistry and regulation. Annu. Rev. Plant Physiol. Plant Mol. Biol. 42, 467–506
Demandre, C., Bahl, J., Serghini, H., Alpha, M.J., Mazliak, P. (1994) Phosphatidylcholine molecular species formed by lysophosphatidylcholine acyltransferase from soya bean microsomes. Phytochemistry 35, 1171–1175
Devor, K.A., Mudd, J.B. (1971) Control of fatty acid distribution in phosphatidylcholine of spinach leaves. J. Lipid Res. 12, 412–419
Dörmann, P., Frentzen, M., Ohlrogge, J.B. (1994) Specificities of the acyl-acyl carrier protein (ACP) thioesterase and glycerol-3-phosphate acyltransferase for octadecenoyl-ACP isomers. Identification of a petroselinoyl-ACP thioesterase in Umbelliferae. Plant Physiol. 104, 839–844
Dutta, P.C., Appleqvist, L.-⫗, Stymne, S. (1992) Utilization of petroselinate (C 18:Δ6) by glycerol acylation enzymes in microsomal preparations of developing embryos of carrot (Daucus carota L.), safflower (Carthamus tinctorius L.) and oil rape (Brassica napus L.). Plant Sci. 81, 57–64
Frentzen, M. (1993) Acyltransferases and triacylglycerols. In: Lipid metabolism in plants, pp. 195–230, Moore, T.S., ed. CRC Press, Boca Raton
Gennity, J.M., Stumpf, P.K. (1985) Studies of the Δ12 desaturase of Carthamus tinctorius L. Arch. Biochem. Biophys. 239, 444–454
Griffiths, G., Stobart, A.K., Stymne, S. (1985) The acylation of sn-glycerol 3-phosphate and the metabolism of phosphatidate in microsomal preparations from the developing cotyledons of safflower (Carthamus tinctorius L.) seed. Biochem. J. 230, 379–388
Griffiths, G., Stymne, S., Stobart, A.K. (1988) The utilization of fatty-acid substrates in triacylglycerol biosynthesis by tissueslices of developing safflower (Carthamus tinctorius L.) and sunflower (Helianthus annuus L.) cotyledons. Planta 173, 309–316
Ichihara, K., Noda, M. (1980) Fatty acid composition and lipid synthesis in developing safflower seeds. Phytochemistry 19, 49–54
Ichihara, K., Asahi, T., Fujii, S. (1987) 1-Acyl-sn-glycerol-3phosphate acyltransferase in maturing safflower seeds and its contribution to the non-random fatty acid distribution of triacylglycerol. Eur. J. Biochem. 167, 339–347
Ichihara, K., Norikura, S., Fujii, S. (1989) Microsomal phosphatidate phosphatase in maturing safflower seeds. Plant Physiol. 90, 413–419
Ichihara, K., Murota, N., Fujii, S. (1990) Intracellular translocation of phosphatidate phosphatase in maturing safflower seeds: a possible mechanism of feedforward control of triacylglycerol synthesis by fatty acids. Biochim. Biophys. Acta 1043, 227–234
Ichihara, K., Nakagawa, M., Tanaka, K. (1993) Acyl-CoA synthetase in maturing safflower seeds. Plant Cell Physiol. 34, 557–566
Lands, W.E.M., Hart, P. (1965) Metabolism of glycerolipids. VI. Specificities of acyl-coenzyme A: phospholipid acyltransferases. J. Biol. Chem. 240, 1905–1911
Lowry, O.H., Rosebrough, N.J., Farr, A.L., Randall, R.J. (1951) Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193, 265–275
Moreau, R.A., Stumpf, P.K. (1982) Solubilization and characterization of an acyl-coenzyme A O-lysophospholipid acyltransferase from the microsomes of developing safflower seeds. Plant Physiol. 69, 1293–1297
Murphy, D.J., Rawsthorne, S., Hills, M.J. (1993) Storage lipid formation in seeds. Seed Sci. Res. 3, 79–95
Rochester, C.P., Bishop, D.G. (1984) The role of lysophosphatidylcholine in lipid synthesis by developing sunflower (Helianthus annuus L.) seed microsomes. Arch. Biochem. Biophys. 232, 249–258
Sanjanwala, M., Sun, G.Y., MacQuarrie, R.A. (1989) Purification and kinetic properties of lysophosphatidylinositol acyltransferase from bovine heart muscle microsomes and comparison with lysophosphatidylcholine acyltransferase. Arch. Biochem. Biophys. 271, 407–413
Sanjanwala, M., Sun, G.Y., Cutrera, M.A., MacQuarrie, R.A. (1988) Acylation of lysophosphatidylcholine in bovine heart muscle microsomes: purification and kinetic properties of acyl-CoA: 1-acyl-sn-glycero-3-phosphocholine O-acyltransferase. Arch. Biochem. Biophys. 265, 476–483
Slack, C.R., Roughan, P.G., Browse, J.A., Gardiner, S.E. (1985) Some properties of cholinephosphotransferase from developing safflower cotyledons. Biochim. Biophys. Acta 833, 438–448
Sperling, P., Heinz, E. (1993) Isomeric sn-1-octadecenyl and sn-2octadecenyl analogues of lysophosphatidylcholine as substrates for acylation and desaturation by plant microsomal membranes. Eur. J. Biochem. 213, 965–971
Stobart, A.K., Stymne, S. (1985a) The interconversion of diacylglycerol and phosphatidylcholine during triacylglycerol production in microsomal preparations of developing cotyledons of safflower (Carthamus tinctorius L.). Biochem. J. 232, 217–221
Stobart, A.K., Stymne, S. (1985b) The regulation of the fatty-acid composition of the triacylglycerols in microsomal preparations from avocado mesocarp and the developing cotyledons of safflower. Planta 163, 119–125
Stymne, S., Stobart, A.K. (1984) Evidence for the reversibility of the acyl-CoA: lysophosphatidylcholine acyltransferase in microsomal preparations from developing safflower (Carthamus tinctorius L.) cotyledons and rat liver. Biochem. J. 223, 305–324
Stymne, S., Stobart, A.K., Glad, G. (1983) The role of the acyl-CoA pool in the synthesis of polyunsaturated 18-carbon fatty acids and triacylglycerol production in the microsomes of developing safflower seeds. Biochim. Biophys. Acta 752, 198–208
Waselake, R.J., Pomeroy, M.K., Furukawa, T.L., Golden, J.L., Little, D.B., Laroche, A. (1993) Developmental profile of diacylglycerol acyltransferase in maturing seeds of oilseed rape and safflower and microspore-derived culture of oilseed rape. Plant Physiol. 102, 565–571
Young, D.L., Lynen, F. (1969) Enzymatic regulation of 3-sn-phosphatidylcholine and triacylglycerol synthesis in states of altered lipid metabolism. J. Biol. Chem. 244, 377–383
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ichihara, K., Mae, K., Sano, Y. et al. 1-Acylglycerophosphocholine O-acyltransferase in maturing safflower seeds. Planta 196, 551–557 (1995). https://doi.org/10.1007/BF00203655
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00203655