Abstract
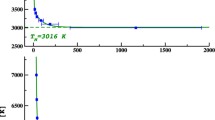
Computer calculations based upon molecular dynamics have enabled us to model the melting behaviour of MgO, a significant component of the Earth's lower mantle. We have successfully employed the super-cell method to study the mechanisms governing the melting process at ambient pressure, and this has enabled us to quantitatively predict values for melting temperature as a function of pressure in the range 0–150 GPa. We have performed melting calculations on a constant stress system containing 1728 ions using a variety of potential models, all of which give a good description of the ambient structural, elastic and defect properties of our system. Our results show that the melting temperature of MgO rises from 2900–3400 K at zero pressure to ∼8000 K at 150 GPa depending upon the potential model used. Our zero pressure results are comparable with previous calculations and close to the experimental value for zero pressure MgO melting of ∼3100 K. We also calculate the melting volume and melting entropy of the system and find our results comparable with zero pressure experimental data for alkali halides but not with the recent high pressure results of Zerr and Boehler (1994) on MgO; the possible sources of this discrepancy are discussed.
Similar content being viewed by others
References
Allen MP, Tildesley DJ (1987) Computer Simulation of Liquids. Clarendon Press, Oxford
Belonoshko AB, Dubrovinsky LS (1995) Molecular Dynamics of NaCl (B 1 and B 2) and MgO (B 1) Melting: Two-Phase Simulation. In press to Am Mineral
Bollmann W (1992) Formation Volume of Schottky Defects (Vacancies) in Inorganic and Organic Compounds and the Defect Formation Mechanism of Melting. Crys Res Technol 27: 673–684
Born M, Huang K (1954) Dynamical Theory of Crystal Lattices. Oxford University Press, Oxford
Catlow CRA, Norgett MJ (1978) United Kingdom Atomic Energy Authority (UKAEA). Report AERE M2936, UKAEA, Harwell
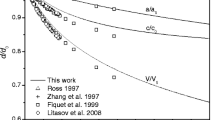
Cohen RE, Gong Z (1994) Melting and Melt Structure of MgO at High Pressures. Phys Rev B 50: 12301–12311
Ewald RP (1921) Die Berechnung Optischer und Elektrostatischer Gitterpotentiale. Ann Phys 64: 253–287
Ewald RP (1937) Gottinger Nechr Math-Phys K1: 55
Ferneyhough R, Fincham D, Price GD, Gillan MJ (1994) The Melting of MgO Studied by Molecular Dynamics Simulation. Model Sim Mat Sci Eng 2: 1101–1110
Fincham D, Mackrodt WC, Mitchell PJ (1994) MgO at High Temperatures and Pressures: Shell Model Lattice Dynamics and Molecular Dynamics. J Phys Cond Matt 6: 393–404
Gear CW (1966) The Numerical Integration of Ordinary Differential Equations of Various Orders. Report ANL 7126, Argonne National Laboratory
Hausleitner C, Hafner J (1989) A Theoretical Study of the Melting Curve of Iron to Very High Pressure. J Phys Cond Matt 1: 5243–5252
Isaak DJ, Cohen RE, Mehl MJ (1990) Calculated Elastic and Thermal Properties of MgO at high Pressure and Temperature. J Geophys Res 95: 7055–7067
Jackson INS, Liebermann RC (1974) Melting and Elastic Shear Instability of Alkali Halides. J Phys Chem Solids 35: 1115–1119
JANAF (1971) Thermochemical Tables 2nd Edition. NSRDS-NBS 37, Washington DC, 1141 pp
Knittle E (1995) Static Compression Measurements of Equations of State. In: Mineral Physics and Crystallography, A Handbook of Physical Constants. AGU Reference Shelf 2. Ed. T.J. Ahrens
Kraut EA, Kennedy GC (1966) New Melting Law at High Pressures. Phys Rev Lett 16: 608–609
Lennard-Jones JE, Devonshire AF (1939) A Theory of Melting and the Structure of Liquid. Proc Roy Soc A 169: 317–318
Lewis GV, Catlow CRA (1985) Potential Models for Ionic Solids. J Phys C Solid State Phys 18: 1149–1161
Lindemann FA (1910) Ueber die Berechnung molekularer Eigenfrequenzen. Phys Z 11: 609–612
Lynden-Bell RM, van Duijneveldt JS, Frenkel D (1993) Free Energy Chenges on Freezing and Melting Ductile Metals. Mol Phys 80: 801–814
Matsui M (1989) Molecular Dynamics Study of the Structural and Thermodynamic Properties of MgO Crystal with Quantum Correction. J Chem Phys 91: 489–494
Matsui M, Price GD (1991) Simulation of the Pre-melting Behaviour of MgSiO3 Perovskite at High Pressures and Temperatures. Nature 351: 735–737
Nose S (1984) A Molecular Dynamics Method for Simulations in the Canonical Ensemble. Mol Phys 52: 255–268
Ohtani E (1983) Melting Temperature Distribution and Fractionation in the Lower Mantle. Phys Earth Planet Int 33: 12–25
Parker SC, Price GD (1989) Computer Modelling of Phase Transitions in Minerals. Adv Solid State Chem 1: 295–327
Parrinello M, Rahman A (1980) Crystal Structure and Pair Potentials: A Molecular Dynamics Study. Phys Rev Lett 45: 1196–1199
Poirier JP (1991) Introduction to the Physics of the Earth's Interior. Cambridge University Press, Cambridge
Poirier JP, Price GD (1992) Dislocation Melting of Metals. Phys Earth Planet Int 69: 153–162
Rivier N, Duffy DM (1982) On the Topological Entropy of Atomic Liquids and the Latent Heat of Fusion. J Phys C Solid State Phys 15: 2867–2874
Simon F, Glatzel G (1929) Bemerkungen zur Schmelzdruckkurve. Z Annorg Allg Chem 178: 309–316
Smith W (1985) MDCSPC Daresbury Laboratory CCP5 program, Daresbury
Stoneham AM, Sangster MJL (1985) The Diffusion of Ions with Multiple Valence — the Oxidation of Transition Metal Alloys. Phil Mag B 52: 717–727
Ubbelohde AR (1978) The Molten State of Matter. Wiley and Sons, Chichister
Winkler B, Dove MT (1992) Thermodynamic Properties of MgSiO3 Perovskite Derived from Large Scale Molecular Dynamics Simulations. Phys Chem Min 18: 407–415
Zerr A, Boehler R (1994) Constraints on the Melting Temperature of the Lower Mantle from High Pressure Experiments on MgO and Magnesiowüstite. Nature 371: 506–508
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Vočadlo, L., Price, G.D. The melting of MgO — computer calculations via molecular dynamics. Phys Chem Minerals 23, 42–49 (1996). https://doi.org/10.1007/BF00202992
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00202992