Abstract
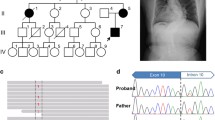
RNase A protection analysis was used in the search for the cause of a non-lethal osteogenesis imperfecta (OI) phenotype (Sillence type III). Cleavage of the hybrid formed between a normal α2(I) sequence and RNA isolated from the patient indicated the presence of a mismatch. The position of the mismatch was determined and the corresponding area of COL1A2 was amplified using the polymerase chain reaction. Sequencing of cloned amplified DNA revealed the deletion, which was not present in either parent, of the final three bases of exon 19 in one of the patient's two COL1A2 alleles. The deletion results in the loss of amino acid 255 (a valine encoded by the last codon of exon 19) of the triple helical region of half of the α2(I) collagen chains but does not disrupt the splicing of the heterogeneous nuclear RNA (hnRNA). This provides further evidence that OI type III may result from autosomal dominant mutations rather than only from autosomal recessive mutations as had previously been believed.
Similar content being viewed by others
References
Armour JAL, Povey S, Jeremiah S, Jeffreys AJ (1990) Systematic cloning of human minisatellites from ordered array charomid libraries. Genomics 8:501–512
Beighton P, Versfeld GA (1985) On the paradoxically high relative prevalence of osteogenesis imperfecta type III in the Black population of South Africa. Clin Genet 27:398–401
Bonadio J, Byers PH (1985) Subtle structural alterations in the chains of type I procollagen produce osteogenesis imperfecta type II. Nature 316:363–366
Bonadio J, Ramirez F, Barr M (1990) An intron mutation in the human α1(I) collagen gene alters the efficiency of pre-mRNA splicing and is associated with osteogenesis imperfecta type II. J Biol Chem 265:2262–2268
Bouche JP (1981) The effect of spermidine on endonuclease inhibition by agarose contaminants. Anal Biochem 115:42–45
Byers PH (1989) Inherited disorders of collagen gene structure and expression. Am J Med Genet 34:72–80
Byers PH (1990) Brittle bones — fragile molecules: disorders of collagen gene structure and expression. Trends Genet 6:293–300
Byers PH, Wallis GA, Willing MC (1991) Osteogenesis imperfecta: translation of mutation to phenotype. J Med Genet 28:433–442
Chomczynski P, Sacchi N (1987) Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 162:156–159
Church GM, Gilbert W (1984) Genomic sequencing. Proc Natl Acad Sci USA 81:1991–1995
Cohn DH, Starman BJ, Blumberg B, Byers PH (1990a) Recurrence of lethal osteogenesis imperfecta due to parental mosaicism for a dominant mutation in a human type I collagen gene. (COL1A1) Am J Hum Genet 46:591–601
Cohn DH, Wallis G, Zhang X, Byers PH (1990b) Serine for glycine substitutions in the α1(I) chain of type I collagen: biological plasticity in the Gly-Pro-Hyp clamp at the carboxyl-terminal end of the triple helical domain. Matrix 10:236
Crouse GF, Frischauf A, Lehrach H (1983) An integrated and simplified approach to cloning into plasmids and single-stranded phages. Method Enzymol 101:78–89
Gibbs RA, Caskey CT (1987) Identification and localization of mutations at the Lesch-Nyhan locus by ribonuclease A cleavage. Science 236:303–305
Hawkins JR, Dalgleish R (1991) The detection and mapping of point mutations by RNase A cleavage. In: Mathew CGP (ed) Methods in molecular biology vol 7: Molecular biology and medicine. Humana Press, Clifton, pp 111–121
Hodges PE, Rosenberg LE (1989) The spf ash mouse: a missense mutation in the ornithine transcarbamylase gene also causes aberrant mRNA splicing. Proc Natl Acad Sci USA 86:4142–4146
Jeffreys AJ, Neumann R, Wilson V (1990) Repeat unit sequence variation in minisatellites: a novel source of DNA polymorphism for studying variation and mutation by single molecule analysis. Cell 60:473–485
Kawasaki ES, Wang AM (1989) Detection of gene expression. In: Erlich HA (ed) PCR technology: Principles and applications for DNA amplification. Stockton Press, New York
Kogan SC, Doherty M, Gitschier J (1987) An improved method for prenatal diagnosis of genetic diseases by analysis of amplified DNA sequences. N Engl J Med 317:985–990
Krawczak M, Cooper DN (1991) Gene deletions causing human genetic disease: mechanisms of mutagenesis and the role of the local DNA sequence environment. Hum Genet 86:425–441
Kuivaniemi H, Sabol C, Tromp G, Sippola-Thiele M, Prockop DJ (1988a) A 19-base pair deletion in the pro-α2(I) gene of type I procollagen that causes in-frame RNA splicing from exon 10 to exon 12 in a proband with atypical osteogenesis imperfecta and his asymptomatic mother. J Biol Chem 263:11407–11413
Kuivaniemi H, Tromp G, Chu M-L, Prockop DJ (1988b) Structure of a full-length cDNA clone for the preproα2(I) chain of human type I procollagen. Biochem J 252:633–640
Kuivaniemi H, Tromp G, Prockop DJ (1991) Mutations in collagen genes: causes of rare and some common diseases in man. FASEB J 5:2052–2060
Mead DA, Szczesna-Skorupa E, Kemper B (1986) Single-stranded DNA ‘blue’ T7 promoter plasmids: a versatile tandem promoter system for cloning and protein engineering. Protein Eng 1:67–74
Miskulin M, Dalgleish R, Kluve-Beckerman B, Rennard SI, Tolstoshev P, Brantly M, Crystal RG (1986) Human type III collagen gene expression is coordinately modulated with the type I collagen genes during fibroblast growth. Biochemistry 25:1408–1413
Murphy G, Ward ES (1989) Sequencing of double stranded DNA. In:Howe CJ, Ward ES (eds) Nucleic acid sequencing: a practical approach. IRL Press, Oxford, New York, Tokyo, pp 99–115
Pack M, Constantinou CD, Kalia K, Neilsen KB, Prockop DJ (1989) Substitution of serine for α1(I)-glycine 844 in a severe variant of osteogenesis imperfecta minimally destabilises the triple helix of type I procollagen. J Biol Chem 264:19694–19699
Pihlajaniemi T, Dickson LA, Pope FM, Korhonen VR, Nicholls A, Prockop DJ, Myers JC (1984) Osteogenesis imperfecta: cloning of a pro-α2(I) collagen gene with a frameshift mutation. J Biol Chem 259:12941–12944
Pingoud A (1985) Spermidine increases the accuracy to type II restriction endonucleases. Eur J Biochem 147:105–109
Prockop DJ (1990) Mutations that alter the primary structure of type I collagen. The perils of a system for generating large structures by the principle of nucleated growth. J Biol Chem 265:15349–15352
Prockop DJ, Constantinou CD, Dombrowski KE, Hojima Y, Kadler KE, Kuivaniemi H, Tromp G, Vogel BE (1989) Type I procollagen: the gene-protein system that harbors most of the mutations causing osteogenesis imperfecta and probably more common heritable disorders of connective tissue. Am J Med Genet 34:60–67
Pruchno CJ, Cohn DH, Wallis GA, Willing MC, Starman BJ, Zhang X, Byers PH (1991) Osteogenesis imperfecta due to recurrent point mutations at CpG dinucleotides in the COL1A1 gene of type I collagen. Hum Genet 87:33–40
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual, 2nd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY
Scheffner M, Munger K, Byrne JC, Howley PM (1991) The state of the p53 and retinoblastoma genes in human cervical carcinoma cell lines. Proc Natl Acad Sci USA 88:5523–5527
Shapiro MB, Senapathy P (1987) RNA splice junctions of different classes of eukaryotes: sequence statistics and functional implications in gene expression. Nucleic Acids Res 15:7155–7174
Short JM, Fernandez JM, Sorge JA, Huse WD (1988) λZap: a bacteriophage λ expression vector with in vivo excision properties. Nucleic Acids Res 16:7583–7600
Sillence DO, Senn A, Danks DM (1979) Genetic heterogeneity in osteogenesis imperfecta. J Med Genet 16:101–116
Sillence DO, Barlow KK, Cole WG, Dietrich S, Garber AP, Rimoin DL (1986) Osteogenesis imperfecta type III. Delineation of the phenotype with reference to genetic heterogeneity. Am J Med Genet 23:821–832
Starman BJ, Eyre D, Charbonneau H, Harrylock M, Weis MA, Weiss L, Graham JM, Byers PH (1989) Osteogenesis imperfecta: The position of substitution for glycine by cysteine in the triple helical domain of the proα1(I) chains of type I collagen determines the clinical phenotype. J Clin Invest 84:1206–1214
Talerico M, Berget SM (1990) Effect of 5′ splice site mutations on splicing of the preceding intron. Mol Cell Biol 10:6299–6305
Tromp G, Prockop DJ (1988) Single base mutation in the proα2(I) collagen gene that causes efficient splicing of RNA from exon 27 to exon 29 and synthesis of a shortened but in frame proα2(I) chain. Proc Natl Acad Sci USA 85:5254–5258
Viljoen D, Beighton P (1987) Osteogenesis imperfecta type III: an ancient mutation in Africa? Am J Med Genet 27:907–912
Wallis GA, Starman BJ, Byers PH (1989) Clinical heterogeneity explained by molecular heterogeneity and somatic mosaicism. Am J Med Genet 45:A228
Wallis GA, Starman BJ, Zinn AB, Byers PH (1990) Variable expression of osteogenesis imperfecta in a nuclear family is explained by somatic mosaicism for a lethal point mutation in the α1(I) gene (COL1A1) of type I collagen in a parent. Am J Med Genet 46:1034–1040
Weil D, D'Alessio M, Ramirez F, de Wet W, Cole WG, Chan D, Bateman JF (1989a) A base substitution in the exon of a collagen gene causes alternative splicing and generates a structurally abnormal polypeptide in a patient with Ehlers-Danlos syndrome type VII. EMBOJ 8:1705–1710
Weil D, D'Alessio M, Ramirez F, Steinmann B, Wirtz MK, Glanville RW, Hollister DW (1989b) Temperature-dependent expression of a collagen splicing defect in the fibroblasts of a patient with Ehlers-Danlos syndrome type VII. J Biol Chem 264:16804–16809
Wenstrup RJ, Willing MC, Starman BJ, Byers PH (1990) Distinct biochemical phenotypes predict clinical severity in nonlethal variants of osteogenesis imperfecta. Am J Hum Genet 46:975–982
Wenstrup RJ, Shrago-Howe AW, Lever LW, Phillips CL, Byers PH, Cohn DH (1991) The effects of different cysteine for glycine substitutions within α2(I) chains. J Biol Chem 266:2590–2594
Willing MC, Cohn DH, Byers PH (1990) Frameshift mutation near the 3′ end of the COL1A1 gene of type I collagen predicts an elongated proα1(I) chain and results in osteogenesis imperfecta type I. J Clin Invest 85:282–290
Winter E, Yamamoto F, Almoguera C, Perucho M (1985) A method to detect and characterise point mutations in transcribed genes: Amplification and overexpression of the mutant c-Ki-ras allele in human tumor cells. Proc Natl Acad Sci USA 82:7575–7579
Wong Z, Wilson V, Patel I, Povey S, Jeffreys AJ (1987) Characterization of a panel of highly variable minisatellites cloned from human DNA. Ann Hum Genet 51:269–288
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Molyneux, K., Starman, B.J., Byers, P.H. et al. A single amino acid deletion in the α2(I) chain of type I collagen produces osteogenesis imperfecta type III. Hum Genet 90, 621–628 (1993). https://doi.org/10.1007/BF00202479
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00202479