Abstract
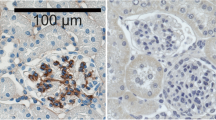
The aim of this study was to examine the distribution of β1 and αv integrins (Ints) and some of their ligands in the kidneys of patients with congenital nephrotic syndrome of the Finnish type (CNF) and in controls using indirect immunofluorescence with monoclonal antibodies. The mesangial reactivity of Int α1 and Int β1 subunits was more variable and an increased glomerular reactivity with Int α3 and Int-α6 antibodies was found in CNF kidneys than in controls. Int α2 subunit was either completely missing from or found in significantly lesser amounts in CNF kidney glomeruli. The immunoreactivity for Int αv was more variable, fainter and also more granular in CNF samples than in control kidneys. The glomerular reactivity for Int β5 was more diffuse and weaker, and in sclerotic Bowman's capsules more intense in CNF kidneys than in controls. Immunoreactivity for Int β6 was restricted and was comparable in extent in CNF and control kidneys. Of the extracellular matrix components studied, the expression of EDAFn, EDBFn, OncFn, Ln α2 chain, Ln β1 chain and tenascin was increased. This is also seen in several glomerular diseases with inflammation and sclerosis. Immunoreactivity for vitronectin was decreased. Several differences were found in the intensity or location of the immunostaining for the β1 and αv Ints and their ligands in CNF kidneys compared with controls, which have not been found in any other proteinuric disease. Disturbed Int expression pattern in CNF may specifically reflect the disturbance of glomerular function caused by the primary defect in this disease.
Similar content being viewed by others
References
Adler S, Eng B (1993) Integrin receptors and function in cultured glomerular endothelial cells. Kidney Int 44:278–284
Ahn C, Tamura R, Yang WS, Lee JS, Quaranta V (1991) Differential expression of integrin α6β1 laminin receptor variants in kidney disease. J Am Soc Nephrol 2:570
Autio-Harmainen H, Rapola J (1983) The thickness of the glomerular basement membrane in congenital nephrotic syndrome of the Finnish type. Nephron 34:48–50
Baraldi A, Furci L, Zambruno G, Rubbiani E, Annessi G, Lusvarghi E (1992) Very late activation-3 integrin is the dominant β1-integrin on the glomerular capillary wall: an immunofluorescence study in nephrotic syndrome. Nephron 62:382–388
Baraldi A, Zambruno G, Furci L, Manca V, Vaschieri C, Lusvarghi E (1994) Beta-1 integrins in the normal human glomerular capillary wall: an immunoelectron microscopy study. Nephron 66:295–301
Breuss JM, Gillett N, Lu L, Sheppard D, Pytela R (1993) Restricted distribution of integrin beta 6 mRNA in primate epithelial tissues. J Histochem Cytochem 41:1521–1527
Bruijn JA, Heer E de (1995) Biology of disease. Adhesion molecules in renal disease. Lab Invest 72:387–394
Cooper HM, Tamura RN, Quaranta V (1991) The major laminin receptor of mouse embryonic stem cells is a novel isoform of the α6β1 integrin. J Cell Biol 115:843–850
Cosio FG (1992) Cell-matrix adhesion receptors: relevance to glomerular pathology. Am J Kidney Dis XX:294–305
Delwel GO, Hogervorst F, Kuikman I, Paulsson M, Timpl R, Sonnenberg A (1993) Expression and function of the cytoplasmic variants of the integrin alpha 6 subunit in transfected K562 cells. Activation-dependent adhesion and interaction with isoforms of laminin. J Biol Chem 268:25865–25875
Delwel GO, Melker AA de, Hogervorst F, Jaspars LH, Fles DLA, Kuikman I, Lindblom A, Paulsson M, Timpl R, Sonnenberg A (1994) Distinct and overlapping ligand specificities of the α3Aβ1 and α6Aβ1 integrins: recognition of laminin isoforms. Mol Biol Cell 5:203–215
Gould VE, Martinez-Lacabe V, Virtanen I, Sahlin KM, Schwartz MM (1992) Differential distribution of tenascin and cellular fibronectins in acute and chronic renal allograft rejection. Lab Invest 67:71–79
Hall DE, Reichardt LF, Crowley E, Holley B, Moezzi H, Sonnenberg A, Damsky CH (1990) The α1/β1 and α6/β1 integrin heterodimers mediate cell attachment to distinct sites on laminin. J Cell Biol 110:2175–2184
Hildebrand J, Schaller M, Parsons JT (1993) Identification of sequences required for the efficient localization of the focal adhesion kinase, pp125FAK, to cellular focal adhesions. J Cell Biol 123:993–1005
Hogervorst F, Admiraal LG, Niessen C, Kuiman I, Janssen H, Daams H, Sonnenberg A (1993) Biochemical characterization and tissue distribution of the A and B variants of the integrin α6 subunit. J Cell Biol 121:179–191
Holmberg C, Antikainen M, Rönnholm K, Ala-Houhala M, Jalanko H (1995) Management of congenital nephrotic syndrome of the Finnish type. Pediatr Nephrol 9:87–93
Hynes R (1992) Integrins: versatility, modulation, and signalling in cell adhesion. Cell 69:11–25
Kanahara K, Yorioka N, Arita M, Ohira N, Yamakido M (1994) Immunohistochemical studies of extracellular matrix components and integrins in IgA nephropathy. Nephron 66:29–37
Kestilä M, Männikkö M, Holmberg C, Gyapay G, Weissenbach J, Savolainen ER, Peltonen L, Tryggvason K (1994) Congenital nephrotic syndrome of the Finnish type maps to the long arm of chromosome 19. Am J Hum Genet 54:757–764
Kestilä M, Männikkö M, Holmberg C, Korpela K, Savolainen ER, Peltonen L, Tryggvason K (1994) Exclusion of eight genes as mutated loci in congenital nephrotic syndrome of the Finnish type. Kidney Int 45:986–990
Köhler G, Milstein C (1975) Continuous cultures of fused cells secreting antibody of pre-defined specificity. Nature 256:495–497
Korhonen M, Ylänne J, Laitinen L, Virtanen I (1990) The α1β6 subunits of integrins are characteristically expressed in distinct segments of developing and adult human nephron. J Cell Biol 111:1245–1254
Korhonen M, Ylänne J, Laitinen L, Virtanen I (1990) Distribution of β1 and β3 integrins in human fetal and adult kidney. Lab Invest 62:616–625
Kriz W, Elger M, Lemley K, Sakai T (1990) Structure of the glomerular mesangium: a biomechanical interpretation. Kidney Int 38 [Suppl 30]:S2–9
Laitinen L, Vartio T, Virtanen I (1991) Cellular fibronectins are differentially expressed in human fetal and adult kidney. Lab Invest 64:492–498
Ljungberg P (1994) Glycosaminoglycans in urine and amniotic fluid in congenital nephrotic syndrome of the Finnish type. Pediatr Nephrol 8:531–536
Ljungberg P, Jalanko H, Holmberg C, Holthofer H (1993) Congenital nephrosis of the Finnish type (CNF): matrix components of the glomerular basement membranes and of cultured mesangial cells. Histochem J 25:606–612
Ljungberg P, Rapola J, Homberg C, Holthöfer H, Jalanko H (1995) Glomerular anionic charge in Congenital nephrotic syndrome of the Finnish type. Histochem J 27:536–546
Ljungberg P, Haltia A, Kuusela P, Jalanko H, Holmberg C, Holthofer H (1995) Noncollagenous matrix components of glomeruli in congenital nephrotic syndrome of the Finnish type (CNF): evidence of abnormal splitting of nidogen in CNF? Exp Nephrol (in press)
Mauch TJ, Vernier RL, Burke BA, Nevins TE (1994) Nephrotic syndrome in the first year of life. In: Holliday MA, Barratt TM, Avner ED (eds) Pediatric nephrology. Williams & Wilkins, Baltimore, pp 788–802
Patey N, Halbwachs-Mecarelli L, Droz D, Lesavre P, Noel LH (1994) Distribution of integrin subunits in normal human kidney. Cell Adhes Commun 2:159–167
Petermann A, Fees H, Grenz H, Goodman SL, Sterzel RB (1993) Polymerase chain reaction and focal contact formation indicate integrin expression in mesangial cells. Kidney Int 44:997–1005
Pöllänen J, Hedman K, Nielsen LS, Dano K, Vaheri A (1988) Ultrastructural localization of plasma membrane-associated urokinase-type plasminogen activator at focal contacts. J Cell Biol 106:87–95
Rapola J (1987) Congenital nephrotic syndrome (Invited review). Pediatr Nephrol 1:441–446
Risteli L, Huttunen NP, Autio-Harmainen H, Risteli J (1982) Slow accumulation of basement membrane collagen in kidney cortex in congenital nephrotic syndrome. Lancet I:712–714
Simon EE, McDonald JA (1990) Extracellular matrix receptors in the kidney cortex. Am J Physiol 259:F783-F792
Sorokin L, Sonnenberg A, Aumailley M, Timpl R, Ekblom P (1990) Recognition of the laminin E8 cell-binding site by an integrin possessing the α6 subunit is essential for epithelial polarization in developing kidney tubules. J Cell Biol 111:1265–1273
Tamura RN, Cooper HM, Collo G, Quaranta V (1991) Cell type-specific integrin variants with alternative α chain cytoplasmic domains. Proc Natl Acad Sci USA 88:10183–10187
Van den Heuvel LPWJ, Van den Born J, Jalanko H, Schröder CH, Veerkamp JH, Assman KJM, Berden JHM, Holmberg C, Rapola J, Monnens LAH (1992) Proteoglycan content of renal basement membranes in the congenital nephrotic syndrome of the Finnish type. Pediatr Nephrol 6:10–15
Vermylen C, Levin M, Mossman J, Barratt TM (1989) Glomerular and urinary heparan sulfate in congenital nephrotic syndrome. Pediatr Nephrol 3:122–129
Vernier RL, Klein DJ, Sisson SP, Mahan JD, Oegema TR, Brown DM (1983) Heparan sulfate-rich anionic sites in the human glomerular basement membrane. Decreased concentration on congenital nephrotic syndrome. N Engl J Med 309:1001–1009
Virtanen I, Laitinen L, Korhonen M (1995) Differential expression of laminin polypeptides in developing and adult human kidney. J Histochem Cytochem 43:621–628
Werb Z, Tremble PM, Behrendtsen O, Crowley E, Damsky CH (1989) Signal transduction through the fibronectin receptor induces collagenase and stromelysin gene expression. J Cell Biol 109:877–889
Yamada KM (1991) Fibronectin and other cell interactive glycoproteins. In: Hay ED (ed) Cell biology of extracellular matrix. Plenum Press, New York, pp 111–146
Yatogho T, Izumi M, Kashiwagi H, Hayashi M (1988) Novel purification of vitronectin from human plasma by heparin affinity chromatography. Cell Struct Funct 13:281–292
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ljungberg, P., Holmberg, C., Jalanko, H. et al. Distribution of renal integrin receptors and their ligands in congenital nephrotic syndrome of the finnish type. Vichows Archiv A Pathol Anat 428, 333–346 (1996). https://doi.org/10.1007/BF00202200
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00202200