Abstract
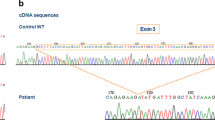
A rare subgroup (approx. 5%) of all chronic granulomatous disease (CGD) patients suffers from mutations in the gene encoding the small p22-phox subunit of the flavocytochrome b 558 heterodimer, the terminal redox component of the phagocyte NADPH oxidase. A male CGD patient with neutrophil granulocytes devoid of any spectrometrically detectable cytochrome b 558 owing to autosomally inherited p22-phox deficiency (phenotype, A22−) is reported. The patient was identified as being compound heterozygous for two independent mutations of his p22-phox alleles. On the maternal allele a single base substitution (A186 to T) was found that predicts a nonconservative replacement of Glu 53 by Val. On his paternal p22-phox allele a G was found to be added to a G stretch between nucleotides G195 and G199 in the cDNA sequence. The resulting frame shift predicts an aberrant open reading frame, 16 amino acids longer than the normal p22-phox polypeptide. Genomic DNA was tested for the presence of the mutant allele by mismatch PCR (polymerase chain reaction). For this purpose, a single base mismatch was introduced at nucleotide position 189, leading to digestion of the normal allele by the restriction enzyme HinfI. The maternal allele was found to be present in 50% of the patient's DNA and in 50% of the DNA from his mother. The same mismatch PCR analysis with control DNA from 35 healthy individuals ruled out the possibility that the single base substitution (A186 to T) represents a common polymorphism. Inheritance of the second allelic mutation (G insertion) was verified by restriction enzyme analysis using BslI [CC(N)7GG] to digest PCR-amplified genomic DNA at the mutation site. PCR in combination with restriction enzyme analysis proved to be a powerful tool for verification of point mutations in the compound heterozygous CGD patient analyzed and may be used for prenatal diagnosis in this family.
Similar content being viewed by others
Abbreviations
- phox :
-
phagocyte oxidase
- CGD:
-
chronic granulomatous disease
- PCR:
-
polymerase chain reaction
References
Beutler E, Kuhl W, Fox M, Tabsh K, Crandall BF (1992) Prenatal diagnosis of glucose-6-phosphate-dehydrogenase deficiency. Acta Haematol 87:103–104
Bohler MC, Seger RA, Mouy R, Vilmer E, Fischer A, Griscelli CA (1986) A study of 25 patients with chronic granulomatous disease: a new classification by correlating respiratory burst, cytochrome b, and flavoprotein. J Clin Immunol 6:136–145
Bolscher BGJM, De Boer M, De Klein A, Weening RS, Roos D (1991) Point mutations in the β-subunit of cytochrome b 558 Leading to X-linked chronic granulomatous disease. Blood 77:2482–2486
Boyum A (1968) Isolation of mononuclear cells and granulocytes from human blood. Scand J Clin Lab Invest 21 [Suppl 97]:77–89
Cha RS, Zarbl H, Keohavong P, Thilly WG (1992) Mismatch amplification mutation assay (MAMA): application to the c-H-ras gene. PCR Methods Appl 2:14–20
Chirgwin JM, Przybyla AE, MacDonald RJ, Rutter WJ (1979) Isolation of biological active ribonucleic acids from sources enriched in ribonuclease. Biochemistry 18:5294–5299
Curnutte JT (1992) Molecular basis of the autosomal recessive forms of chronic granulomatous disease. Immunodeficiency Rev 3:149–172
Curnutte JT, Whitten DM, Babior BM (1974) Defective superoxide production by granulocytes from patients with chronic granulomatous disease. N Engl J Med 290:593–597
De Boer M, De Klein A, Hossle JP, Seger R, Corbeel L, Weening RS, Roos D (1992) Cytochrome b 558-negative, autosomal recessive chronic granulomatous disease: two new mutations in the cytochrome b 558 light chain of the NADPH oxidase (p22-phox). Am J Hum Genet 51:1127–1135
Dinauer MC, Pierce EA, Bruns GAP, Curnutte JT, Orkin SH (1990) Human neutrophil cytochrome b light chain (p22-phox) gene structure, chromosomal location, and mutations in cytochrome-negative autosomal recessive chronic granulomatous disease. J Clin Invest 86:1729–1737
Dinauer MC, Pierce EA, Erickson RW, Mühlebach TJ, Messner H, Orkin SH, Seger RA, Curnutte JT (1991) Point mutation in the cytoplasmic domain of the neutrophil p22-phox cytochrome b subunit is associated with a nonfunctional NADPH oxidase and chronic granulomatous disease. Proc Natl Acad Sci USA 88:11231–11235
Jacobson D, Moskovits T (1991) Rapid nonradioactive screening for activating ras oncogene mutations using PCR-primer introduced restriction analysis (PCR-PIRA). PCR Methods Appl 1: 146–148
Kuppuswamy MN, Hoffmann JW, Kasper CK, Spritzer SSG, Goce SL, Bajaj SP (1991) Single nucleotide primer extension to detect genetic diseases: Experimental application to hemophilia B (factor IX) and cystic fibrosis genes. Proc Natl Acad Sci USA 88:1143–1147
Lutter R, Van Schaik ML, Van Zwieten R, Wever R, Roos D (1985) Purification and partial characterization of the b-type cytochrome from human polymorphonuclear leukocytes. J Biol Chem 260:2237–2244
Maeda M, Bawlw EV, Kulkarni R, Beutler E, Yoshida A (1992) Molecular abnormalities of a phosphoglycerate kinase variant generated by spontaneous mutation. Blood 79:2759–2762
Mizuno Y, Hara T, Nakamura M, Ueda K, Minakami S, Take H (1988) Classification of chronic granulomatous disease on the basis of monoclonal antibody-defined surface cytochrome b deficiency. J Pediatr 113:548
Mühlebach TJ, Erny C, Suter R, Brägger C, Yalman N, Kobayashi S, Nakamura M, Seger RA (1991) Analysis of various types of chronic granulomatous disease with the monoclonal antibody 7D5 directed against the small subunit of surface cytochrome b558. Pediatr Allergy Immunol 2:124–130
Parkos CA, Dinauer MC, Walker LE, Allen RA, Jesaitis AJ, Orkin SH (1988) Primary structure and unique expression of the 22-kilodalton light chain of human neutrophil cytochrome b. Proc Natl Acad Sci USA 85:3319–3323
Preisig E, Hitzig WH (1971) Nitroblue-tetrazolium test for the detection of chronic granulomatous disease. Eur J Clin Invest 1:409–412
Roos D, De Boer M (1986) Purification and cryopreservation of phagocytes from human blood. Methods Enzymol 132:225–243
Seger RA, Berthet F, Hossle JP (1992) Chronic granulomatous disease. Pediatr Allergy Immunol 3:1–10
Smith RM, Curnutte JT (1991) Molecular basis of chronic granulomatous disease. Blood 77:673–686
Verhoeven AJ, Bolscher BG, Meerhof LJ, Van Zwieten R, Keijer J, Weening RS, Roos D (1989) Characterization of two monoclonal antibodies against cytochrome b 558 of human neutrophils. Blood 73:1686–1692
Verlaan-de Vries M, Boogaard ME, Van den Elst H, Van Boom JH, Van der Eb AJ, Bos JL (1986) A dot blot screening procedure for mutated ras oncogenes using synthetic oligonucleotides. Gene 50:313–320
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hossle, J.P., de Boer, M., Seger, R.A. et al. Identification of allele-specific p22-phox mutations in a compound heterozygous patient with chronic granulomatous disease by mismatch PCR and restriction enzyme analysis. Hum Genet 93, 437–442 (1994). https://doi.org/10.1007/BF00201671
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00201671