Summary
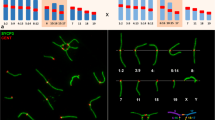
The segregation products of the Rb(6.16) translocation were studied at first cleavage metaphase. Male mice heterozygous for the translocation mated at 3- and 14-day intervals to superovulated random-bred ICR females. Chromosome preparations of the recovered one-cell embryos were sequentially G- and C-banded and male and female complements analyzed cytogenetically. Of the 309 zygotes analyzed from both mating groups, no unbalanced segregants of the translocation were observed. In the 3-day group there was a highly significant difference (P<0.001) from the expected 1:1 ratio between sperm with normal (70.5%) and balanced segregants (26.2%) of alternate segregation. In the 14-day group the level of significance for the difference was ten times lower (P<0.01) as normal segregants were observed in 56.4% of the sperm and balanced ones in 36.5%. A comparison of the two groups using a 2×2 contingency table showed that segregant type was related to mating frequency (P<0.05). There was a highly significant distortion (P<0.01) of the sex ratio, with 178 Y-bearing and 131 X-bearing sperm in the combined populations. When sex ratio was analyzed according to mating intervals, the distortion was significant (P<0.01) only for the 3-day group. An analysis of the sex ratio according to the segregant type showed no significant deviation from 1:1 for type 1 segregants, which had normal chromosomes, while type 2 segregants, with the translocation, had a deficiency of X-bearing sperm. This deficiency was significant (P<0.05) only for the 3-day population. Analysis of meiotic preparations showed no association between the translocation trivalent and the X-Y bivalent. Our results, obtained under physiological conditions, provide definitive evidence for sperm selection and support previous findings that aging of sperm can modify the effect of selection.
Similar content being viewed by others
References
Bond DJ, Chandley AC (1983) Aneuploidy. (Oxford monographs on medical genetics) Oxford University Press, Oxford
Boué A (1982) Antenatal diagnosis of chromosomal disorders from research to public health application. In: Bonne-Tamir B, Cohen T, Goodman RH (eds) Progress in clinical and biological research, vol 103B: Human genetics, part B: Medical aspects. (Proceedings of the Sixth International Congress of Human Genetics, Jerusalem 1981) Liss, New York, pp 523–531
Boué A, Gallano P (1984) Collaborative study of the segregation of inherited chromosome structural rearrangements in 1356 prenatal diagnosis. Prenat Diagn 4:45–67
Bruére AN, Scott IS, Henderson LM (1981) Aneuploid spermatocyte frequency in domestic sheep heterozygous for three Robertsonian translocation. J Reprod Fertil 63:61–66
Bucán M, Yang-Feng T, Coberg-Poley AM, Wolgemuth DJ, Guenet J-L, Franke U, Lehrach H (1986) Genetic and cytogenetic localization of the homeobox containing genes on mouse chromosome 6 and human chromosome 7. EMBO J 5:2899–2905
Buckland RA, Evans HJ, Sumner AT (1971) Identifying mouse chromosomes with the ASG technique. Exp Cell Res 69:231–236
Committee on Standardized Genetic Nomenclature for Mice (1972) Standard karyotype of the mouse, Mus musculus. J Hered 63:69–71
Erickson RP, Lewis SE, Butley M (1981) Is haploid gene expression possible for sperm antigens? J Reprod Immunol 3:195–217
Evans EP, Lyon MF, Daglish M (1967) A mouse translocation giving a metacentric marker chromosome. Cytogenetics 6:105–119
Ford CE, Evans EP (1973) Robertsonian translocations in mice: segregational irregularities in male heterozygotes and zygotic imbalance. In: Wahrman J, Lewis KR (eds) Chromosome today, vol 4. Wiley, New York, pp 387–397
Gropp A, Winking H (1981) Robertsonian translocations: cytology, meiosis, segregation patterns and biological consequences of heterozygosity. Symp Zool Soc Lond 47:141–181
Guichaoua MR, Devictor M, Toga-Piquet C, Luciani JM, Stahl A (1989) Meiotic technique. In: Verma RS, Babu A (eds) Human chromosomes manual of basic techniques. Pergamon Press, New York, p 30
Hamerton JL (1970) Robertsonian translocations: evidence on segregation from family studies. In: Jacobs PA, Price WH, Law P (eds) Human population cytogenetics. Edinburgh University Press, Edinburgh, pp 63–80
Hecht NB (1990) Regulation of “haploid-expressed genes” in the male germ cells. J Reprod Fertil 88:679–693
Martin RH (1988) Cytogenetic analysis of sperm from a male heterozygous for a 13;14 Robertsonian translocation. Hum Genet 80:357–361
Martin-DeLeon PA, Boice ML (1982) Sperm aging in the male and cytogenetic anomalies: an animal model. Hum Genet 62:70–77
Martin-DeLeon PA, Boice ML (1983) Spontaneous heteroploidy in one-cell mouse embryos. Cytogenet Cell Genet 35:57–63
Martin-DeLeon PA, Boice ML (1985) Sperm aging in the male after sexual rest: contribution to chromosome anomalies. Gamete Res 12:151–163
Martin-DeLeon PA, Williams MB (1987) Sexual behavior and Down syndrome: the biological mechanism. Am J Med Genet 27:693–700
Martin-DeLeon PA, Shaver EL, Gammal EB (1973) Chromosome abnormalities in rabbit blastocysts resulting from spermatozoa aged in the male tract. Fertil Steril 24:212–219
Olds-Clarke P (1984) Genetic analysis of mammalian spermatogenesis: use of the t complex in the mouse in studies of spermatogenesis and sperm function. Ann NY Acad Sci 438:206–216
Palmiter RD, Wilkie TM, Chen HY, Brinter RL (1984) Transmission distortion and mosaicism in an unusual transgenic mouse pedigree. Cell 36:869–877
Pellestor F (1990) Analysis of meiotic segregation in a man heterozygous for a 13;15 Robertsonian translocation and a review of the literature. Hum Genet 85:49–54
Pellestor F, Sele B, Jalbert H (1987) Chromosome analysis of spermatozoa from a male heterozygous for a 13;14 Robertsonian translocation. Hum Genet 76:116–120
Propst F, Rosenberg MP, Oskarsson MK, Russell LB, Chi Nguyen-Huu M, Nadeau J, Jenkins NA, Copeland NG, Vande Woude GF (1988) Genetic analysis and developmental regulation of testis-specific RNA expression of Mos, Abl, actin and Hox-1. 4. Oncogene 2:227–233
Rappold GA, Stubbs L, Labert S, Crkvenjakov RB, Lehrach H (1987) Identification of a testis-specific gene from the mouse t-complex next to a CpG-rich island. EMBO J 6:1975–1980
Tarkowski AK (1966) An air-drying method of chromosome preparations from mouse eggs. Cytogenetics 5:394–400
Wilkie TM, Palmiter RD (1987) Analysis of the integrant in Myk-103 transgenic mice in which males fail to transmit the integant. Mol Cell Biol 7:1646–1655
Zackowski JL, Martin-DeLeon PA (1989) Segregation products of male mice doubly heterozygous for the Rb(6. 16) and Rb(16. 17) translocations: influence of sperm karyotype on fertilizing competence under varying mating frequencies. Gamete Res 22:93–107
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Aranha, I.P., Martin-DeLeon, P.A. The murine Rb(6.16) translocation: evidence for sperm selection and a modulating effect of aging. Hum Genet 87, 278–284 (1991). https://doi.org/10.1007/BF00200904
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00200904