Summary
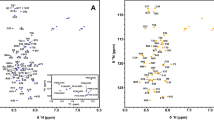
Oncostatin M (OM) is a cytokine that shares a structural and functional relationship with interleukin-6, leukemia inhibitory factor, and granulocyte-colony stimulating factor, which regulate the proliferation and differentiation of a variety of cell types. A mutant version of human OM in which two N-linked glycosylation sites and an unpaired cysteine have been mutated to alanine (N76A/C81A/N193A) has been expressed and shown to be active. The triple mutant has been doubly isotope-labeled with 13C and 15N in order to utilize heteronuclear multidimensional NMR techniques for structure determination. Approximately 90% of the backbone resonances were assigned from a combination of triple-resonance data (HNCA, HNCO, CBCACONH, HBHACONH, HNHA and HCACO), intraresidue and sequential NOEs (3D 15N-NOESY-HMQC and 13C-HSQC-NOESY) and side-chain information obtained from the CCONH and HCCONH experiments. Preliminary analysis of the NOE pattern in the 15N-NOESY-HMQC spectrum and the 13Cα secondary chemical shifts predicts a secondary structure for OM consisting of four α-helices with three intervening helical regions, consistent with the four-helix-bundle motif found for this cytokine family. As a 203-residue protein with a molecular weight of 24 kDa, Oncostatin M is the largest α-helical protein yet assigned.
Similar content being viewed by others
References
Abdel-Meguid, S.S., Shieh, H.S., Smith, W.W., Dayringer, H.E., Violand, B.N. and Bentle, L.A. (1987) Proc. Natl. Acad. Sci. USA, 84, 6434–6437.
Barton, B.E., Jackson, J.V., Lee, F. and Wagner, J. (1994) Cytokine, 6, 147–153.
Bax, A. and Pochapsky, S.S. (1992) J. Magn. Reson., 99, 638–643.
Bazan, J.F. (1990) Immunol. Today, 11, 350–354.
Boulton, T.G., Stahl, N. and Yancopoulos, G.D. (1994) J. Biol. Chem., 269, 11648–11655.
Brown, T.J., Rowe, J.M., Shoyab, M. and Gladstone, P. (1990) UCLA Symp. Mol. Cell. Biol., New Ser., 131, 195–206.
Brown, T.J., Rowe, J.M., Liu, J. and Shoyab, M. (1991) J. Immunol., 147, 2175–2180.
Brown, T.J., Liu, J., Brashem-Stein, C. and Shoyab, M. (1993) Blood, 82, 33–37.
Bruce, A.G., Hoggatt, I.H. and Rose, T.M. (1992a) J. Immunol., 149, 1271–1275.
Bruce, A.G., Linsley, P.S. and Rose, T.M. (1992b) Prog. Growth Factor Res., 4, 157–170.
Cai, J., Gill, P.S., Masood, R., Chandrasoma, P., Jung, B., Law, R.E. and Radka, S.F. (1994) Am. J. Pathol., 145, 74–79.
Clore, G.M. and Gronenborn, A.M. (1989) Crit. Rev. Biochem. Mol. Biol., 24, 479–564.
Clore, G.M. and Gronenborn, A.M. (1991) Annu. Rev. Biophys. Biophys. Chem., 20, 29–63.
DeVos, A.M., Ultsch, M. and Kossiakoff, A.A. (1992) Science, 255, 306–312.
Delaglio, F., Grzesiek, S., Vuister, G.W., Zhu, G., Pfeifer, J. and Bax, A. (1995) J. Biomol. NMR, 6, 277–293.
Fogh, R.H., Schipper, D., Boelens, R. and Kaptein, R. (1994) J. Biomol. NMR, 4, 123–128.
Fogh, R.H., Schipper, D., Boelens, R. and Kaptein, R. (1995) J. Biomol. NMR, 5, 259–270.
Friedrichs, M.S., Mueller, L. and Wittekind, M. (1994) J. Biomol. NMR, 4, 703–726.
Garrett, D.S., Powers, R., Gronenborn, A.M. and Clore, G.M. (1991) J. Magn. Reson., 95, 214–220.
Gearing, D.P. and Bruce, A.G. (1992) New Biologist, 4, 61–65.
Grzesiek, S. and Bax, A. (1992a) J. Am. Chem. Soc., 114, 6291–6293.
Grzesiek, S. and Bax, A. (1992b) J. Magn. Reson., 96, 432–440.
Grzesiek, S. and Bax, A. (1993) J. Biomol. NMR, 3, 185–204.
Grzesiek, S., Anglister, J. and Bax, A. (1993a) J. Magn. Reson. Ser. B, 101, 114–119.
Grzesiek, S., Anglister, J., Ren, H. and Bax, A. (1993b) J. Am. Chem. Soc., 115, 4369–4370.
Horn, D., Fitzpatrick, W.C., Gompper, P.T., Ochs, V., Bolton-Hanson, M., Zarling, J., Malik, N., Todaro, G.J. and Linsley, P.S. (1990) Growth Factors, 22, 157–165.
Kallestad, J.C., Shoyab, M. and Linsley, P.S. (1991) J. Biol. Chem., 266, 8940–8945.
Kay, L.E., Ikura, M., Tschudin, R. and Bax, A. (1990) J. Magn. Reson., 89, 496–514.
Landon, M. (1977) Methods Enzymol., 47, 145–149.
Liu, J., Modrell, B., Aruffo, A., Scharnowske, S. and Shoyab, M. (1994) Cytokine, 6, 272–278.
Marion, D., Driscoll, P.C., Kay, L.E., Wingfield, P.T., Bax, A., Gronenborn, A.M. and Clore, G.M. (1989a) Biochemistry, 28, 6150–6156.
Marion, D., Ikura, M., Tschudin, R. and Bax, A. (1989b) J. Magn. Reson., 85, 393–399.
Marion, D., Kay, L.E., Sparks, S.W., Torchia, D.A. and Bax, A. (1989c) J. Am. Chem. Soc., 111, 1515–1517.
Miles, S.A., Martinez-Maza, O., Rezai, A., Magpantay, L., Kishimoto, T., Nakamura, S., Radka, S.F. and Linsley, P.S. (1992) Science, 255, 1432–1434.
Nair, B.C., DeVico, A.L., Nakamura, S., Copeland, T.D., Chen, Y., Patel, A., O'Neil, T., Oroszlan, S., Gallo, R.C. and Sarngadharan, M.G. (1992) Science, 255, 1430–1432.
Nishimoto, N., Ogata, A., Shima, Y., Tani, Y., Ogawa, H., Nakagawa, M., Sugiyama, H., Yoshizaki, K. and Kishimoto, T. (1994) J. Exp. Med., 179, 1343–1347.
Powers, R., Gronenborn, A.M., Clore, G.M. and Bax, A. (1991) J. Magn. Reson., 94, 209–213.
Powers, R., Garrett, D.S., March, C.J., Frieden, E.A., Gronenborn, A.M. and Clore, G.M. (1992a) Biochemistry, 31, 4334–4346.
Powers, R., Garrett, D.S., March, C.J., Frieden, E.A., Gronenborn, A.M. and Clore, G.M. (1992b) Science, 256, 1673–1677.
Prickett, K.S., Amberg, D.C. and Hopp, T.P. (1989) Biotechniques, 7, 580–589.
Remerowski, M.L., Domke, T., Groenewegen, A., Pepermans, H.A.M., Hilbers, C.W. and Van deVen, F.J.M. (1994) J. Biomol. NMR, 4, 257–278.
Richards, C.D., Brown, T.J., Shoyab, M., Baumann, N. and Gauldie, J. (1992) J. Immunol., 148, 1731–1736.
Robinson, R.C., Grey, L.M., Staunton, D., Vankelecom, H., Vernallis, A.B., Moreau, J.F., Stuart, D.I., Heath, J.K. and Jones, E.Y. (1994) Cell, 77, 1101–1116.
Rose, T.M. and Bruce, A.G. (1991) Proc. Natl. Acad. Sci. USA, 88, 8641–8645.
Rose, T.M., Weiford, D.M., Gunderson, N.L. and Bruce, A.G. (1994) Cytokine, 6, 48–54.
Seavey, B.R., Farr, E.A., Westler, W.M. and Markley, J.L. (1991) J. Bionol. NMR, 1, 217–230.
Sklenář, V., Piotto, M., Leppik, R. and Saudek, V. (1993) J. Magn. Reson. Ser. A, 102, 241–245.
Spera, S. and Bax, A. (1991) J. Am. Chem. Soc., 113, 5490–5492.
Sporeno, E., Paonessa, G., Salvati, A.L., Graziani, R., Delmastro, P., Ciliberto, G. and Toniatti, C. (1994) J. Biol. Chem., 269, 10991–10995.
Tanigawa, T., Nicola, N., McArthur, G.A., Strasser, A. and Begley, C.G. (1995) Blood, 85, 379–390.
Vuister, G.W. and Bax, A. (1993) J. Am. Chem. Soc., 115, 7772–7777.
Yamazaki, T., Lee, W., Arrowsmith, C.H., Muhandiram, D.R. and Kay, L.E. (1994a) J. Am. Chem. Soc., 116, 11655–11666.
Yamazaki, T., Lee, W., Revington, M., Mattiello, D.L., Dahlquist, F.W., Arrowsmith, C.H. and Kay, L.E. (1994b) J. Am. Chem. Soc., 116, 6464–6465.
Zarling, J.M., Shoyab, M., Marquardt, H., Hanson, M.B., Lioubin, M.N. and Todaro, G.J. (1986) Proc. Natl. Acad. Sci. USA, 83, 9739–9743.
Author information
Authors and Affiliations
Additional information
To whom correspondence should be addressed.
Rights and permissions
About this article
Cite this article
Hoffman, R.C., Moy, F.J., Price, V. et al. Resonance assignments for Oncostatin M, a 24-kDa α-helical protein. J Biomol NMR 7, 273–282 (1996). https://doi.org/10.1007/BF00200429
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00200429



