Summary
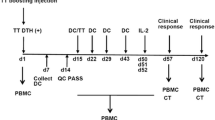
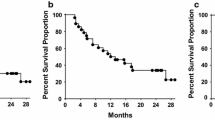
The ability of active specific immunotherapy to enhance immune responses to autologous tumor-associated antigens (TAA) and to prolong the disease-free interval was evaluated in patients with Dukes B2 and C colorectal carcinoma who had undergone potentially curative resections. Patients were sensitized in the early postoperative period with irradiated autologous adenocarcinoma cells mixed with bacillus Calmette-Guérin (BCG) to yield either a low-dose vaccine (3×106 tumor cells) or a high-dose vaccine (1×107 tumor cells). Six of seven patients who received the low-dose vaccine developed delayed-type hypersensitivity (DTH) responses to autologous tumor cells upon completion of the vaccination, whereas all four patients receiving high-dose vaccine displayed a positive DTH response. However, DTH responses to autologous TAA waned within 3 months in all patients receiving the low-dose vaccine; DTH responses persisted for 3 months in three of the four high-dose vaccine patients. In vitro lymphoproliferative responses to TAA correlated with DTH responses to autologous tumor cells. Active specific immunotherapy appeared to induce specific immune responses either in vitro or in vivo to autologous TAA because it did not induce responses to autologous mucosa cells. There were no complications caused by BCG or tumor cells. This series demonstrates that active specific immunotherapy is a nontoxic treatment that augments immunity to autologous TAA.
Similar content being viewed by others
References
Astler VB, Coller FA (1954) The prognostic significance of direct extension of carcinoma of the colon and rectum. Ann Surg 139:846–852
Bhide SV, Maru GB, Sawai MM, Ranadine KJ (1978) Isoniazid tumorigenicity in mice under different experimental conditions. Int J Cancer 21:318–326
Boyum A (1968) Isolation of mononuclear cells and granulocytes from human blood. Scand J Clin Lab Invest 21:77–86
Filipe MI, Branfoot AC (1974) Abnormal patterns of mucus secretion in apparently normal mucosa of large intestine with carcinoma. Cancer 34:282–290
Gastrointestinal Tumor Study Group (1984) Adjuvant therapy of colon cancer-results of a prospectively randomized trial. New Engl J Med 320:737–743
Hanna Jr MG, Peters LC (1978) Specific immunotherapy of established visceral micrometastases by BCG-tumor cell vaccine alone or as an adjuvant to surgery. Cancer 42:2613–2625
Hanna Jr MG, Brandhorst JC, Peters LC (1979) Active specific immunotherapy of residual micrometastases. An evaluation of sources, doses, and ratios of BCG with tumor cells. Cancer Immunol Immunother 7:165–173
Hanna Jr MG, Pollack VA, Peters LC, Hoover HC (1982) Active specific immunotherapy of established micrometastases with BCG plus tumor cell vaccines. Cancer 49:659–664
Holden HT, Oldham RK, Ortaldo, JR, Herberman RB (1976) Cryopreservation of the functional reactivity of normal and immune leukocytes and of tumor cells. In: Bloom BR, David JR (eds) In vitro methods in cell-mediated and tumor immunity. Academic Press, New York pp 723–729
Hoover Jr HC, Surdyke M, Dangel R, Peters LC, Hanna Jr, MG (1984) Delayed cutaneous hypersensitivity to autologous tumor cells in colorectal cancer patients immunized with an autologous tumor cell: Bacillus Calmette-Guerin vaccine. Cancer Res 44:1671–1676
Hoover Jr HC, Surdyke MG, Dengel RB, Peters LC, Hanna Jr MG (1985) Prospectively randomized trial of adjuvant active specific immunotherapy for human colorectal cancer. Cancer 55:1236–1243
Lev R, Grover R (1981) Precursors of human colon carcinoma: a serial section study of colectomy specimens. Cancer 47:2007–2015
Peters LC, Brandhorst JC, Hanna Jr MG (1979) Preparation of immunotherapeutic autologous tumor cell vaccines from solid tumors. Cancer Res 39:1353–1360
Silverberg E (1983) Cancer statistics. CA 33:9–25
Vanky F, Stjernsward J (1976) Lymphocyte stimulation test for detection of tumor-specific reactivity in humans. In: Bloom BR, David JR (eds) In vitro methods in cell-mediated and tumor immunity Academic Press, New York pp 597–606
Author information
Authors and Affiliations
Additional information
This project was supported by grants from Cutter Laboratories, Inc., the Annual Campaign of the University of Texas System Cancer Center, and the National Cancer Cytology Center
Rights and permissions
About this article
Cite this article
Jessup, J.M., McBride, C.M., Ames, F.C. et al. Active specific immunotherapy of Dukes B2 and C colorectal carcinoma: Comparison of two doses of the vaccine. Cancer Immunol Immunother 21, 233–239 (1986). https://doi.org/10.1007/BF00199367
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00199367