Abstract
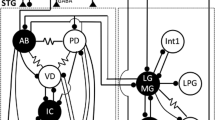
The neurotransmitters mediating the synaptic interactions in the pyloric system of the stomatogastric ganglion of a stomatopod, Squilla oratoria, were examined. Putative transmitters were applied iontophoretically to the pyloric cells. Glutamate and GABA produced inhibitory responses in all motoneurons but acetylcholine did not. These inhibitory responses were due to increases in conductance to either K+ or Cl− or both, and blocked by picrotoxin. The inhibitory postsynaptic potentials evoked by the constrictor and dilator neurons were different in their time courses, reversal potentials, ion selectivities, and picrotoxin sensitivities. Glutamate is a transmitter candidate for inhibitory synapses made among the pyloric cells as well as for their neuromuscular junctions. In some cells, glutamate and acetylcholine evoked excitatory responses which were blocked by joro spider toxin and by tubocurare, respectively. They mediated the extrinsic inputs to modulate the pyloric rhythm. The transmitter, glutamate, is conserved in the ganglion neurons between stomatopods and decapods during evolution. Use of two transmitters, glutamate and acetylcholine, may have evolved in decapods, while the ionic mechanism is preserved in both orders. The neuromodulators, acetylcholine and γ-aminobutyric acid, are conserved between both orders. Glutamate may be used as the neuromodulator in stomatopods.
Similar content being viewed by others
Abbreviations
- ACh:
-
acetylcholine
- EPSP:
-
excitatory postsynaptic potential
- GABA:
-
γ-aminobutyric acid
- Glu:
-
glutamate
- IC:
-
inferior cardiac
- IPSP:
-
inhibitory postsynaptic potential
- JSTX:
-
joro spider toxin
- LP:
-
lateral pyloric
- pcp:
-
posterior cardiac plate
- PTX:
-
picrotoxin
References
Benson JA (1988) Transmitter receptors on insect neuronal somata: GABAergic and cholinergic pharmacology. In: Lunt GG (ed) The molecular basis of drug and pesticide action — Neurotox '88. Elsevier Biomedical, Amsterdam, pp 193–206
Bidaut M (1980) Pharmacological dissection of pyloric network of the lobster stomatogastric ganglion using picrotoxin. J Neurophysiol 44:1089–1101
Cazalets JR, Cournil I, Geffard M, Moulins M (1987) Suppressive control of oscillatory activity in crustacean pyloric neurons: Implication of GABAergic inputs. J Neurosci 7:2884–2893
Chiba C, Tazaki K (1992) Glutamatergic motoneurons in the stomatogastric ganglion of the mantis shrimp Squilla oratoria. J Comp Physiol A 170:773–786
Cull-Candy SG (1976) Two types of extra-junctional L-glutamate receptors in locust muscle fibres. J Physiol (Lond) 255:449–464
Dickinson PS, Nagy F (1983) Control of a central pattern generator by an identified modulatory interneurone in Crustacea. II. Induction and modification of plateau properties in pyloric neurons. J Exp Biol 105:59–82
Eisen JS, Marder E (1982) Mechanisms underlying pattern generation in lobster stomatogastric ganglion as determined by selective inactivation of identified neurons. III. Synaptic connections of electrically coupled pyloric neurons. J Neurophysiol 48:1392–1415
Eisen JS, Marder E (1984) A mechanism for the production of phase shifts in a pattern generator. J Neurophysiol 51:1375–1393
Gerschenfeld HM (1973) Chemical transmission in invertebrate central nervous systems and neuromuscular junctions. Physiol Rev 53:1–119
Harris-Warrick RM, Nagy F, Nusbaum MP (1992) Neuromodulation of stomatogastric networks by identified neurons and transmitters. In: Harris-Warrick RM, Marder E, Selverston AI, Moulins M (eds) Dynamic biological networks. The stomatogastric nervous system. MIT Press, Boston, pp 87–137
Hooper SL, O'Neil MB, Wagner R, Ewer J, Golowasch J, Marder E (1986) The innervation of the pyloric region of the crab, Cancer borealis: Homologous muscles in decapod species are differently innervated. J Comp Physiol A 159:227–240
Katz PS (1991) Neuromodulation and the evolution of a simple motor system. Sem Neurosci 3:379–389
Katz PS, Tazaki K (1992) Comparative and evolutionary aspects of the crustacean stomatogastric system. In: Harris-Warrick RM, Marder E, Selverston AI, Moulins M (eds) Dynamic biological networks. The stomatogastric nervous system. MIT Press, Boston, pp 221–261
Kawai N, Niwa A, Abe T (1982) Spider venom contains specific receptor blocker of glutaminergic synapses. Brain Res 247:169–171
Kunze JC (1981) The functional morphology of stomatopod Crustacea. Phil Trans R Soc London B 292:255–318
Lingle C (1980) The sensitivity of decapod foregut muscles to acetylcholine and glutamate. J Comp Physiol 138:187–199
Lingle C, Auerbach A (1983) Comparison of excitatory currents activated by different transmitters on crustacean muscle. II. Glutamate-activated currents and comparison with acetylcholine currents present on the same muscle. J Gen Physiol 81:571–588
Marder E (1974) Acetylcholine as an excitatory neuromuscular transmitter in the stomatogastric system of the lobster. Nature 251:730–731
Marder E (1976) Cholinergic motor neurons in the stomatogastric system of the lobster. J Physiol (Lond) 257:63–86
Marder E (1987) Neurotransmitters and neuromodulators. In: Selverston AI, Moulins M (eds) The crustacean stomatogastric system. A model for the study of central nervous systems. Springer, Berlin Heidelberg New York, pp 263–300
Marder E, Eisen JS (1984) Transmitter identification of pyloric neurons: Electrically coupled neurons use different transmitters. J Neurophysiol 51:1345–1361
Marder E, Paupardin-Tritsch D (1978) The pharmacological properties of some crustacean neuronal acetylcholine, γ-aminobutyric acid, and L-glutamate responses. J Physiol (Lond) 280:213–236
Marder E, Paupardin-Tritsch D (1980) Picrotoxin block of a depolarizing ACh response. Brain Res 181:223–227
Mat Jais AM, Kerkut GA, Walker RJ (1983) The ionic mechanisms associated with the biphasic glutamate response on leech Retzius cells. Comp Biochem Physiol C 74:115–126
Maynard DM (1972) Simpler networks. Ann NY Acad Sci 193:59–72
Maynard DM, Dando MR (1974) The structure of the stomatogastric neuromuscular system in Callinectes sapidus, Homarusamericanus and Panulirus argus. Phil Trans R Soc London B 268:161–220
Maynard DM, Walton KD (1975) Effects of maintained depolarization of presynaptic neurons on inhibitory transmission in lobster neuron. J Comp Physiol 97:215–243
Nagy F, Dickinson PS (1983) Control of a central pattern generator by an identified modulatory interneurone in Crustacea. I. Modulation on the pyloric motor output. J Exp Biol 105:33–58
Russell DF, Hartline DK (1981) A multiaction synapse evoking both EPSPs and enhancement of endogenous bursting. Brain Res 223:19–38
Schram FR (1982) The fossil record and evolution of Crustacea. In: Abele LG (ed) The biology of Crustacea. Vol. I. Systematics, the fossil record and biogeography. Academic Press, New York, pp 93–147
Selverston AI, Moulins M (1987) The crustacean stomatogastric system. A model for the study of central nervous system. Springer, Berlin Heidelberg New York, 338 pp
Takeuchi A, Takeuchi N (1972) Actions of transmitter substances on the neuromuscular junctions of vertebrate and invertebrates. Adv Biophysics 3:45–95
Tazaki K (1988) The anatomy and physiology of the stomatogastric nervous system of Squilla. II. The cardiac system. Zool Sci 5:299–309
Tazaki K (1993) Motor pattern generation of the posterior cardiac plate-pyloric system in the stomatogastric ganglion of the mantis shrimp Squilla oratoria. J Comp Physiol A 172:369–387
Tazaki K, Chiba C (1991) Mechanisms underlying burst generation of the pyloric muscle in the mantis shrimp Squilla oratoria. J Comp Physiol A 169:737–750
Tazaki K, Chiba C (1993) Cellular properties and modulation of the stomatogastric ganglion neurons of a stomatopod, Squilla oratoria. J Comp Physiol A 173:85–101
Tazaki K, Miyazaki T (1991) Neural control of the posterior cardiac plate and pyloric regions of the mantis shrimp Squilla oratoria: Neurogenic and myogenic activities of muscles. J Comp Physiol A 168:265–279
Tazaki K, Miyatani M, Ando F (1986) The anatomy and physiology of the stomatogastric nervous system of Squilla. I. The posterior cardiac plate and the pyloric systems. J Comp Physiol A 159:521–533
Wafford KA, Sattelle DB (1989) L-glutamate receptors on the cell body membrane of an identified insect motor neurone. J Exp Biol 144:449–462
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Tazaki, K., Chiba, C. Glutamate, acetylcholine, and γ-aminobutyric acid as transmitters in the pyloric system of the stomatogastric ganglion of a stomatopod, Squilla oratoria . J Comp Physiol A 175, 487–504 (1994). https://doi.org/10.1007/BF00199256
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00199256