Abstract
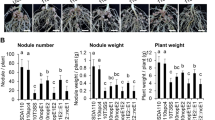
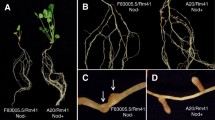
Rhizobium fredii USDA257 does not nodulate McCall soybean (Glycine max (L.) Merr.), but two transposon-mutants derived from it, 257DH4 and 257DH5, do. All three organisms cause curling of McCall root hairs and induce the formation of underlying cortical cell divisions. The mutants produce infection threads, and many of the meristematic foci develop into nodules. In contrast, root hairs that deform in response to USDA257 lack infection threads, and meristematic activity ceases prior to the appearance of nodule meristems. Root systems nodulated by mutant 257DH4 reduce acetylene at rates similar to those of roots nodulated by reference R. fredii strain USDA191. The presence of living cells of USDA257 in inocula leads to strong inhibition of nodulation by 257DH4 but not by 257DH5. This blocking effect depends on the ratio of USDA257 cells to 257DH4 cells in the inoculum; nodules that form contain cells of 257DH4, but not those of parental strain USDA257.
Similar content being viewed by others
References
Appelbaum, E.R., Chartrain, N., Thompson, D., Johansen, K. O'Connell, M., McLoughlin, T. (1985a) Genes of Rhizobium japonicum involved in development of nodules. In: Nitrogen fixation research progress, pp. 101–107, Evans, H.J., Bottomley, P.H., eds. Nijhoff, Dordrecht, The Netherlands
Appelbaum, E.R., Johansen, K., Chartrain, N. (1985b) Symbiotic mutants of USDA191, a fast-growing Rhizobium that nodulates soybeans. Mol. Gen. Genet. 201, 454–461
Appelbaum, E.R., McLoughlin, T.J., O'Connell, M., Chartrain, N. (1985c) Expression of symbiotic genes of Rhizobium japonicum USDA 191 in other rhizobia. J. Bacteriol. 163, 385–388
Broughton, W.J., Samrey, U., Bohlool, B.B. (1982) Competition for nodulation of Pisum sativum cv. Afghanistan requires live rhizobia and a plant component. Can. J. Microbiol. 28, 162–168
Calvert, H.E., Pence, M.K., Pierce, M., Malik, N.S.A., Bauer, W.D. (1984) Anatomical analysis of the development and distribution of Rhizobium infections in soybean roots. Can. J. Bot. 62, 2375–2384
Davis, E.O., Evans, I.J., Johnston, A.W.B. (1988) Identification of nodX, a gene that allows Rhizobium leguminosarum biovar viciae strain TOM to nodulate Afghanistan peas. Mol. Gen. Genet. 212, 531–535
Debellé, F., Rosenberg, C., Vasse, J., Maillet, F., Martinez, E., Dénarié, J., Truchet, G. (1986) Assignment of symbiotic developmental phenotypes to common and specific nodulation (nod) genetic loci of Rhizobium meliloti. J. Bacteriol. 168, 1075–1086
Djordjevic, M.A., Schofield, P.R., Rolfe, B.G. (1985) Tn5 mutagenesis of Rhizobium trifolii host-specific nodulation genes result in mutants with altered host-range ability. Mol. Gen. Genet. 200, 463–471
Dowdle, S.F., Bohlool, B.B. (1985) Predominance of fast-growing Rhizobium japonicum in a soybean field in the People's Republic of China. Appl. Environ. Microbiol. 50, 1171–1176
Dowling, D.N., Stanley, J., Broughton, W.J. (1989) Competitive nodulation blocking of Afghanistan pea is determined by no-dABC and nodFE alleles in Rhizobium leguminosarum. Mol. Gen. Genet. 216, 170–174
Downie, J.A., Knight, C.D., Johnston, A.W.B., Rossen, L. (1985) Identification of genes and gene products involved in the nodulation of peas by Rhizobium leguminosarum. Mol. Gen. Genet. 198, 255–262
DuTeau, N.M., Palmer, R.G., Atherly, A.G. (1986) Fast-growing Rhizobium fredii are poor nitrogen-fixing symbionts of soybean. Crop Sci. 26, 884–889
Faucher, C., Maillet, F., Vasse, J., Rosenberg, C., van Brussel, A.A.N., Truchet, G., Dénarié, J. (1988) Rhizobium meliloti host range nodH gene determines production of an alfalfa-specific extracellular signal. J. Bacteriol. 170, 5489–5499
Hattori, J., Johnson, D.A. (1984) Fast-growing Rhizobium japonicum that effectively nodulates several commercial Glycine max L. Merrill cultivars. Appl. Environ. Microbiol. 48, 234–235
Heron, D.S., Pueppke, S.G. (1984) Mode of infection, nodulation specificity, and indigenous plasmids of 11 fast-growing Rhizobium japonicum strains. J. Bacteriol. 160, 1061–1066
Heron, D.S., Pueppke, S.G. (1987) Regulation of nodulation in the soybean-Rhizobium symbiosis. Strain and cultivar specificity. Plant Physiol. 84, 1391–1396
Heron, D.S., Érsek, T., Krishnan, H.B., Pueppke, S.G. (1989) Nodulation mutants of Rhizobium fredii USDA257. Molec. Plant-Microbe Interact. 2, 4–10
Horvath, B., Barabas, L, Wieneke, U., Schell, J., Kondorosi, A. (1986) Organization, structure and symbiotic function of Rhizobium meliloti nodulation genes determining host specificity for alfalfa. Cell 46, 335–343
Israel, D.W., Mathis, J.N., Barbour, W.M., Elkan, G.H. (1986) Symbiotic effectiveness and host-strain interactions of Rhizobium fredii USDA 191 on different soybean cultivars. Appl. Environ. Microbiol. 51, 898–903
Keyser, H.H., Bohlool, B.B., Hu, T.S., Weber, D.F. (1982) Fast-growing rhizobia isolated from root nodules of soybean. Science 215, 1631–1632
Lie, T.A. (1984) Host genes in Pisum sativum L. conferring resistance to European Rhizobium leguminosarum strains. Plant Soil 82, 415–425
Lie, T.A., Winarno, R., Timmermans, P.C.J.M. (1978) Rhizobium strains isolated from wild and cultivated legumes: Suppression of nodulation by a non-nodulating Rhizobium strain. In: Microbial Ecology, pp. 398–401, Loutit, M.W., Miles, J.A.R., eds. Springer-Verlag, Berlin
Lie, T.A., Nijland, G.J., Waluyo, S.H. (1988) Competition between nodulating and non-nodulating Rhizobium strains: delay of nodulation. In: Physiological limitations and the genetic improvement of symbiotic nitrogen fixation, pp 127–136, O'Gara, F., Manian, S., Drevon, J.J., eds. Kluwer Academic Publishers, Dordrecht, The Netherlands
Lin, J., Walsh, K.B., Johnson, D.A., Canvin, D.T., Shujin, W., Layzell, D.B. (1987) Characterization of R. fredii QB1130, a strain effective on commercial soybean cultivars. Plant Soil 99, 441–446
Mort, A.J., Bauer, W.D. (1980) Composition of the capsular and extracellular polysaccharides of Rhizobium japonicum. Plant Physiol. 66, 158–163
Pueppke, S.G. (1983) Rhizobium infection threads in root hairs of Glycine max (L.) Merr., Glycine soja Sieb, et Zucc., and Vigna unguiculata (L.) Walp. Can. J. Microbiol. 29, 69–76
Pueppke, S.G. (1986) Nodule distribution on legume roots: specificity and response to the presence of soil. Soil Biol. Biochem. 18, 601–606
Pueppke, S.G., Payne, J.H. (1987) Responses of Rj 1 and rj 1 soybean isolines to inoculation with Bradyrhizobium japonicum. Plant Physiol. 84, 1291–1295
Sato, T. (1967) A modified method for lead staining of thin sections. J. Electron Microsc. 16, 133
Scholla, M.H., Elkan, G.H. (1984) Rhizobium fredii sp. nov., a fast-growing species that effectively nodulates soybeans. Int. J. Syst. Bacteriol. 34, 484–486
Schwinghamer, E.A., Evans, H.J., Dawson, M.D. (1970) Evaluation of effectiveness in mutant strains of Rhizobium by acetylene reduction relative to other criteria for N2 fixation. Plant Soil 33, 192–212
Spurr, A.R. (1969) A low viscosity epoxy resin embedding medium for electron microscopy. J. Ultrastruct. Res. 26, 31–43
Turgeon, B.G., Bauer, W.D. (1982) Early events in the infection of soybean by Rhizobium japonicum. Time course and cytology of the initial infection process. Can. J. Bot. 6, 152–161
Vincent, J.M. (1970) A manual for the practical study of root-nodule bacteria. Blackwell, Oxford, UK
Winarno, R., Lie, T.A. (1979) Competition between Rhizobium strains in nodule formation: interaction between nodulating and non-nodulating strains. Plant Soil 51, 135–142
Author information
Authors and Affiliations
Additional information
This research was underwritten by Grant No. 88-37234-4101 from the U.S. Department of Agriculture. Asita Chatterjee was supported by funds from the Food for the 21st Century Program of the University of Missouri, and Pedro Balatti was supported by the Consejo Nacional de Investigaciones Cientificas Tecnicas de la Republica Argentina. We thank W.J. Broughton for providing facilities during preparation of the manuscript. This is Journal Series No. 10857 of the Missouri Agricultural Experiment Station.
Rights and permissions
About this article
Cite this article
Chatterjee, A., Balatti, P.A., Gibbons, W. et al. Interaction of Rhizobium fredii USDA257 and nodulation mutants derived from it with the agronomically improved soybean cultivar McCall. Planta 180, 303–311 (1990). https://doi.org/10.1007/BF00198781
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00198781