Abstract
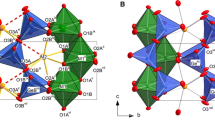
Synthetic almandine garnet, Fe3Al2Si3O12, has been studied by temperature — dependent single crystal X-ray diffraction and57Fe Mössbauer spectroscopy. The Fe2+ doublet in almandine is characterized by a small asymmetry between the high and low-velocity peaks that decreases in magnitude with decreasing temperature from 420 to 15 K. The X-ray results show that the Fe2+ cation is dynamically disordered with an anisotropic motion within the eight-coordinated site in garnet. The magnitudes of the X-ray determined mean-square-vibrational amplitudes of this motion parallel,x ∥, and perpendicular,x ⊥, to the principle axes of the electric field gradient give a calculated angular dependence of the electric quadrupole interaction of theI 1/2 toI 3/2 transitions that agree with the experimentally measured peak ratios. This is the first recognition of anisotropic recoil free fraction of57Fe in silicates.
Similar content being viewed by others
References
Amthauer G, Annersten H, Hafner SS (1976) The Mössbauer spectrum of57Fe in silicate garnets. Z Kristallogr 143:14–55
Armbruster Th (1986) Crystal structure refinement and thermal expansion of a Li, Na, Be-cordierite between 100 and 550 K. Z Kristallogr 174:205–217
Armbruster Th, Bürgi HB, Kunz M, Gnos E, Brönnimann S, Lienert C (1990) Variation of displacement parameters in structure refinements of low albite. Amer Mineral 75:135–140
Armbruster Th, Geiger CA, Lager GA (1992) Single crystal X-ray structure study of synthetic pyrope-almandine garnets at 100 and 293 K. Amer Mineral 77:518–527
Bancroft GM, Maddock AG, Burns RG (1967) Application of the Mössbauer effect to silicate mineralogy. I. Iron silicates of known crystal structure. Geochim Cosmochim Acta 31:2219–2246
Born L, Zemann J (1964) Abstandsberechnungen und gitterenerge-ische Berechnungen an Granaten. Beitr Mineral Petrog 10:2–23
Chandrasekhar K, Bürgi HB (1984) Dynamic processes in crystals examined through difference vibrational parametersΔU: The low-spin-high-spin transition in Tris(dithiocarbamato)iron(III) complexes. Acta Crystallogr B40:387–397
Enraf-Nonius (1983) Structure determination package (SDP). Enraf-Nonius, Delft, The Netherlands
Geiger CA, Brearley A, Amthauer G, Ross CRII (in preparation) Synthesis of almandine garnet and characterization by57Fe Mössbauer and Single crystal FTIR spectroscopy, powder X-ray refinement and transmission electron microscopy: The role of defects
Gol'danskii VI, Makarov EF, Khrapov VV (1963) On the difference in two peaks of Quadrupole splitting in Mössbauer spectra. Phys Lett 3:344–346
Gol'danskii VI (1964) The Mössbauer effect and its applications in chemistry. Van Nostrand, New York
Gonser K (1975) From a strange effect to Mössbauer spectroscopy. In: Gonser K (ed) Topics in Applied Physics, vol 5, pp 1–51. Mössbauer Spectroscopy. Springer, Berlin Heidelberg NewYork
Greenwood NN, Gibb TC (1971) Mössbauer spectroscopy pp 75–76. Chapman and Hall, London
Herber RH, Chandra S (1970) Gol'danskii-Karyagin effect in Dimethyl Tin Difluoride. J Chem Phys 52:6045–6048
Hirshfeld FL (1976) Can X-ray data distinguish bonding effects from vibrational smearing? Acta Crystallogr A 32:239–344
Hummel W, Hauser J, Bürgi HB (1990) PEANUT a computer-graphics program to represent atomic displacement parameters. J Mol Graph 8:214–220
Kunz M, Armbruster Th (1990) Difference displacement parameters in alkali feldspars: Effects of (Si, Al) order-disorder. Amer Mineral 75:141–149
Lyubutin IS, Dodokin AP (1971) Temperature dependence of the Mössbauer effect for Fe2+ in dodecahedral coordination in garnet. Sov Phys Crystallogr 15:1091–1092
Menzer G (1928) Die Kristallstruktur der Granate. Z Kristallogr 69:300–396
Murad E, Wagner FE (1987) The Mössbauer spectrum of almandine. Phys Chem Minerals 14:264–269
Novak GA, Gibbs GV (1971) The crystal chemistry of the silicate garnets. Am Mineral 56:791–825
Prandl W (1971) Die magnetische Struktur und die Atomparameter des Almandins Al2Fe3(SiO4)3. Z Kristallogr 134:333–343
Prandl W, Wagner F (1971) Die Orientierung des elektrischen Feld-gradienten und das innere Magnetfeld beim Almandin. Z Kristallogr 134:344–349
Prandl W (1971) Die magnetische Struktur und die Atomparameter des Almandins Al2Fe3(SiO4)3. Z Kristallogr 134:333–343
Seiler P, Schweizer WB, Dunitz JD (1984) Parameter refinement for tetrafluorotherephthalonitrile at 98 K: making the best of a bad job. Acta Crystallogr B40:319–327
Zucker UH, Perenthaler E, Kuhs WF, Bachmann R, Schulz H (1983) PROMETHEUS: A program system for investigation of anharmonic thermal vibrations in crystals. J Appl Crystallogr 16:358
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Geiger, C.A., Armbruster, T., Lager, G.A. et al. A combined temperature dependent57Fe Mössbauer and single crystal X-ray diffraction study of synthetic almandine: evidence for the Gol'danskii-Karyagin effect. Phys Chem Minerals 19, 121–126 (1992). https://doi.org/10.1007/BF00198609
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00198609