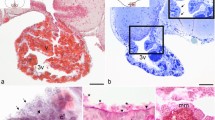
Abstract
This study has evaluated the development of the hypothalamic vasopressin system and nephrons of the kidney in desert rodents, Meriones shawi, which effectively retain water by excretion of highly concentrated urine. The vasopressin system was studied immunocyto chemically at the 18th fetal day, at the 2nd, 13th, 27th postnatal days and in adulthood. The kidneys were investigated at the 2nd, 13th postnatal days and in adulthood using microdissection technique. Occasional vasopressin-immunoreactive neurons were observed as early as the 18th fetal day, only in the paraventricular nucleus. From the 2nd postnatal day onwards, vasopressin neurons increased progressively in number, being mainly concentrated in the supraoptic and paraventricular nuclei, as well as in the ventral retrochiasmatic region. Transient neuronal populations were also observed at the 13th postnatal day in the lateral preoptic area and anterior hypothalamic nucleus. Apart from the neurons, the glandular cells of the tuberal lobe showed immunostaining from the 18th fetal day, the first age studied, until the 13th postnatal day. The fibers of differentiating vasopressin neurons grew towards the circumventricular/neurohemal organs, terminating in the organum vasculosum of the lamina terminalis and the lateral ventricles as early as the 18th fetal day, as well as the third ventricle, the posterior lobe and the external zone of the median eminence between the 2nd and 13th postnatal days. The kidney in 2 day-old Meriones comprised nephrons at different stages of development from an S-shaped body to well-differentiated nephrons. At the 13th postnatal day, as in adulthood, the nephrons were well differentiated and characterized by long, thin loops descending to different levels of papilla. Thus, according to our morphological data the hypothalamic vasopressin neurons and nephrons in the kidney of Meriones reach the definitive state by the end of the 2nd postnatal week.
Similar content being viewed by others
References
Alstein M, Gainer H (1988) Differential biosynthesis and posttranslational processing of vasopressin and oxytocin in rat brain during embryonic and postnatal development. J Neurosci 8:3967–3977
Ang VTY, Jenkins JS (1984) Neurohypophysial hormones in the adrenal medulla. J Clin Endocrinol Metabol 58:688–691
Baddouri K, El Hilali M (1986) Sécrétion de l'hormone antidiurétique et fonction rénale au cours du réveil de l'hibernation de Jaculus orientalis. J Physiol (Paris) 81:202–208
Baddouri K, Marchetti J, Hilali M, Menard J (1981) Mesure de l'hormone antidiurétique et de l'activité rénine plasmatique chez les Rongeurs désertiques (Jaculus orientalis et Jaculus deserti). C R Acad Sci 292:143–146
Baddouri K, Butlen D, Imbert-Teboul M, Le Bouffant F, Marchetti J, Chabardès D, Morel F (1984) Plasma antidiuretic hormone levels and kidney responsiveness to vasopressin in the Jerboa, Jaculus orientalis. Gen Comp Endocrinol 54:203–215
Baddouri K, El Hilali M, Lachiver F (1985) Capacité d'économie en eau chez une espèce de rongeur désertique: Jaculus orientalis. Mammalia 49:543–549
Boer GJ (1987) Development of vasopressin systems and their functions. In: Gash DM, Boer GJ (eds) Vasopressin, principles and properties. Plenum Press, New York, pp 117–174
Boer GJ, Snijdewint FGM, Swaab DF (1988) Neuropeptides and functional neuroteratology. Prog Brain Res 73:245–264
Buijs RM (1978) Intra and extrahypothalamic vasopressin and oxytocin pathways in the rat. Cell Tissue Res 192:423–435
Buijs RM (1992) The development of vasopressin and oxytocin systems in the brain. In: Björklund A, Hökfelt T, Tohyama M (eds) Handbook of chemical neuroanatomy, vol 10. Ontogeny of transmitters and peptides in the CNS. Elsevier, Amsterdam, pp 547–570
Butlen D, Baddouri K, Rajerison RM, Guillon G, Cantau B, Jard S (1984) Plasma antidiuretic hormone levels and liver vasopressin receptors in the Jerboa, Jaculus orientalis, and rat. Gen Comp Endocrinol 54:216–229
Caffé AR, Leeuwen FW van (1983) Vasopressin-immunoreactive cells in the dorsomedial hypothalamic region, medial amygdaloid nucleus and locus coeruleus of the rat. Cell Tissue Res 233:23–33
Castel M, Gainez H, Dellmann HD (1984) Neuronal secretory activity. Int Rev Cytol 88:304–459
Choy VJ, Watkins WB (1979) Maturation of the hypothalamo neurohypophysial system. Localization of neurophysin, oxytocin and vasopressin in the hypothalamus and neural lobe of developing rat brain. Cell Tissue Res 197:325–336
Compoint C (1968) Etude du diencéphale de Meriones shawi (Gerbillidé) en comparaison avec celui de Rattus norvegicus albinos (Muridé). J Hirnforsch 10:325–350
De Rouffignac C, Bankir L (1990) L'économie de l'eau chez les mammifères. La Recherche 21:654–665
Devries GJ, Ruijs RM, Swaab DF (1981) Ontogeny of vasopres sinergic neurons of the supraoptic nucleus and their extrahypothalamic projections in the rat brain-presence of sex difference in the lateral septum. Brain Res 218:67–78
Dicker SE (1970) Mechanisms of urine concentration and dilution in mammals. Arnold, London
Dlouha H, Krecek J, Zicha J (1982) Postnatal development and diabetes insipidus in Brattleboro rats. Ann NY Acad Sci 394:10–19
Doucet A, Barlet C, Baddouri K (1987) Effect of water intake on Na+-K+-ATPase in nephron segm of the desert rodent, Jaculus orientalis. Pflügers Arch 408:129–132
Fahrenholz F, Jurzak M, Gerstbenger R, Haase W (1993) Renal and central vasopressin receptors: immunocytochemical localization. Ann NY Acad Sci 689:194–206
Goncharevskaya OA (1977) Midcortical and juxtamedullary nephrons during postnatal ontogenesis of the rat. Arch Anat Histol Embryol 72:20–26
Goncharevskaya OA, Dlouhá H (1975) The development of various nephron generations during postnatal ontogenesis in the rat. Anat Rec 182:367–375
Hatton GI (1990) Emerging concepts of structure-function dynamics in adult brain: the hypothalamo-neurohypophysial system. Prog Neurobiol 34:437–504
Hewitt S (1981) Plasticity of renal function in the Australian desert rodent Notomys alexis. Comp Biochem Physiol 69A:297–304
Hyodo S, Yamada C, Takezawa T, Urano A (1992) Expression of provasopressin gene during ontogeny in the hypothalamus of developing mice. Neuroscience 46:241–250
Ivanova LN, Natochin YuV, Serebryakov EP, Goncharevskaya OA, Knyazkova LG, Lavrova EA, Nasledova NI, Pechurkina NI, Podsekayeva GV, Shakhmatova EI (1980) Comparative study of the concentrating mechanism in the kidney of the big gerbil (Rhombomys opimus L.) and the water vole (Arvicola terrestris L.). Comp Biochem Physiol 66:499–505
Kriz W, Bankir L (1982) ADH-induced changes in the epithelium of the thick ascending limb in Brattleboro rats with hereditary hypothalamic diabetes insipidus. Ann NY Acad Sci 394:424–432
Kriz W, Bankir L (1988) A standard nomenclature for structures of the kidney. Kidney Int 33:1–7
Kriz W, Kaissling B (1992) Structural organization of the mammalian kidney. In: Seidin DW, Giebisch G (eds) The kidney: physiology and pathophysiology. Raven Press, New York pp 707–777
Laurent FM, Hindelang C, Klein MJ, Stoeckel ME, Felix JM (1989) Expression of the oxytocin and vasopressin genes in the rat hypothalamus during development: an in situ hybridization study. Dev Brain Res 46:145–154
Lazcano MA, Bentura ML, Toledano A (1990) Morphometric study on the development of magnocellular neurons of the supraoptic nucleus utilising immunocytochemical methods. J Anat 168:1–11
MacMillen RE, Lee AK (1967) Australian desert mice: independence of exogenous water. Science 158:383–385
McNicol AM, Murray JE, McMeekin W (1990) Vasopressin stimulation of cell proliferation in the rat pituitary gland in vitro. J Endocrinol 126:255–259
Moore RY (1991) Development of the suprachiasmatic nucleus. In: Klein DC, Moore RY, Reppert SM (eds) Suprachiasmatic nucleus. The mind's clock. Oxford University Press, New York, pp 391–404
Natochin YuV (1994) Vasopressin: a search for the mechanism of increased water permeability. Sov Sci Rev F Physiol Gen Biol 7:85–147
Natochin YuV, Meshehrskii IG, Goncharevskaya OA, Makarenko IG, Shakhmatova El, Ugrumov MV, Feoktistova MYu, Alonso G (1994) Comparative study on the osmoregulatory system in the hamsters Phodopus roborovskii and Rhodopus sungarus. J Evol Biochem Physiol 30:344–357
Nielsen S, DiGiovanni SR, Christensen EI, Knepper MA, Harris HW (1993) Cellular and subcellular immunolocalization of vasopressin-regulated water channel in rat kidney. Proc Natl Acad Sci USA 90:11663–11667
Reppert SM, Uhl GR (1987) Vasopressin messenger ribonucleic acid in supraoptic and suprachiasmatic nuclei: appearance and circadian regulation during development. Endocrinology 120:2483–2487
Reppert SM, Uhl GR (1988) The vasopressin gene is expressed prior to stimulation in the supraoptic nuclei in fetal rats. Brain Res 456:392–396
Schmidt-Nielsen K, Schmidt-Nielsen B (1952) Water metabolism of desert mammals. Physiol Rev 32:135–165
Siga E, Horster M (1991) Regulation of osmotic water permeability during differentiation of inner medullary collecting duct. Am J Physiol 260:F710-F716
Spitzer A, Schwartz G (1992) The kidney during development. In: Windhager EE (ed) Handbook of Physiology, Section 8. Renal physiology, vol 1. Oxford University Press, New York, pp 475–544
Swaab DF (1980) Neuropeptides and brain development a working hypothesis. In: Di Benedetta C, et al. (eds) Multidisciplinary approach to brain development. Elsevier, Amsterdam, pp 181–196
Trembleau A, Ugrumov MV, Roche D, Calas A (1995) Vasopressin and oxytocin gene expressions in intact rats and under catecholamine deficiency during ontogenesis. Brain Res Bull 37:437–448
Trinh-Trang-Tan MM, Boudy N, Doute M and Bankir L (1984) Effect of long and short-term antidiuretic hormone availability in internephron heterogeneity in adult rat. Am J Physiol 246:F879-F878
Wathes DC (1984) Possible actions of gonadal oxytocin and vasopressin. J Reprod Fertil 71:315–345
Whitnall MH, Key S, Ben-Barak Y, Ozato K, Gainer H (1985) Neurophysin in the hypothalamo-neurohypophysial system. II. Immunocytochemical studies of the ontogeny of oxytocinergic and vasopressinergic neurons. J Neurosci 5:98–109
Yamashita T, Kawamoto K, Kawashima S (1988) Arginine vasopressin contents of the hypothalamus and pituitary during fetal and postnatal development in the mouse. Dev Growth Different 30:563–571
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Rabhi, M., Ugrumov, M.V., Goncharevskaya, O.A. et al. Development of the hypothalamic vasopressin system and nephrons in Meriones shawi during ontogenesis. Anat Embryol 193, 281–296 (1996). https://doi.org/10.1007/BF00198331
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00198331