Abstract
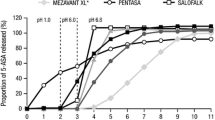
One gram single dose of Pentasa controlled-release capsules was administered to 24 healthy volunteers under fasting condition. Mean plasma 5-aminosalicylic acid (5-ASA) and acetyl 5-ASA concentrations peaked at 0.53 μg · ml−1 and 1.33 μg · ml−1 from 3 to 4 hours following dosing, respectively. The half-lives of both compounds could not be determined as absorption of 5-ASA was continuous throughout the gastrointestinal tract. An average of 29.4% (CV: 27%) of the dose was excreted in the urine primarily as acetyl 5-ASA. Up to 91.1% of the dose was released from the capsules. Forty percent of the dose (CV: 40%) was eliminated in the feces, with 8.9% of the dose remained as formulation bounded 5-ASA, indicating that controlled-release capsules continue to release drug throughout the GI tract. 5-ASA contributed 46.7% of the salicylates eliminated in the feces and acetyl 5-ASA accounted for the balance. Controlled-release capsules produced three times more total salicylates and 10 times more total and free 5-ASA in the feces than did 5-ASA suspension. Thus, while lower systemic levels of salicylates were absorbed, greater therapeutic quantities of 5-ASA were available in the bowel.
Similar content being viewed by others
References
Elliot R (1981) Crohn's disease: drug therapy, diet or surgery. Drugs 21:383–389
Northfield TC (1977) Ulcerative colitis and Crohn's colitis. Differential diagnosis and treatment. Drugs 14:198–206
Peppercorn MA (1984) Sulfasalazine - pharmacology, clinical use, toxicity, and related new drug development. Annals of Internal Medicine 3:377–386
Das KM, Dubin R (1976) Clinical pharmacokinetics of sulphasalazine. Clinical Pharmacokinetics 1:406–425
Singleton JW, Law DH, Kelley ML Jr, Makjhian HS, Sturdeveant RAL (1979) National cooperative Crohn's disease study: adverse reactions to study drug. Gastroenterology 77:870–882
Taffet SL, Das KM (1983) Sulfasalazine - adverse effects and desensitization. Digestive Diseases Sciences 28:833–842
Toovey S, Hudson E, Hendry WF, Levi AJ (1981) Sulphasalazine and male infertility: reversibility and possible mechanism. Gut 22:445–451
Yu DK, Elvin AT, Morrill B, et al (1990) Effect of food coad-ministration on 5-aminosalicylic acid oral suspension bioavailability. Clin Pharmacol Ther 48:26–33
Ryan K, Bottom C, Cameron C, Payne P, Guernsey B (1988) Effect of pH on dissolution of various mesalamine mesalamine dosage forms (abstract). Gastroenterology 94:5,A391.17
Hanauer S, Beshears L, Wilkinson C, et al (1990) Induction of remission in a dose-ranging study of oral mesalamine capsules (Pentasa(R)) (abstract). Gastroenterology 98:5,A174
Hanauer S, Belker ME, Gitnick G, et al (1990) Multi-center, placebo-controlled, dose-ranging study of oral Pentasa (controlled-release mesalamine) for active Crohn's disease: Preliminary results (abstract). Gastroenterology 98:5,A173
Law R, Hanauer S, Rick G, et al (1990) Multi-center, open-label, long-term study of oral Pentasa(R) (controlled-release mesalamine) in Crohn's disease (abstract). Gastroenterology 98:5,A185
Beshears L, Hanauer S, Guernsey B, et al (1990) Open-label study of oral mesalamine capsules (Pentasa(R)) for the treatment of ulcerative colitis (abstract). Gastroenterology 98:5, A159
Gibaldi M, Perrier D (1982) Pharmacokinetics, 2nd edn. Dekker, New York, pp 409
Davis SS (1986) Evaluation of the gastrointestinal transit of pharmaceutical dosage forms using the technique of gamma scintigraphy. Sciences Pharmacetutiques Rev 2:1015–1022
Tjornelund J, Hansen S, Cornett C (1989) New metabolites of the drug 5-aminosalicylic acid. I: N-B-D-glucopyranosyl-5-aminosalicylic acid. Xenobiotica 19:891–899
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Yu, D.K., Morrill, B., Eichmeier, L.S. et al. Pharmacokinetics of 5-aminosalicylic acid from controlled-release capsules in man. Eur J Clin Pharmacol 48, 273–277 (1995). https://doi.org/10.1007/BF00198311
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00198311