Abstract
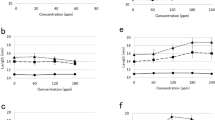
The interaction of free IAA and its amino acid conjugates on growth and development of cultured tomato hypocotyl tissue (Lycopersicon esculentum Mill. cv. Marglobe) was studied. In a nutrient medium containing 10 μmol/L of benzyladenine, free IAA stimulated shoot and root development with little callus proliferation. In contrast, all IAA-amino acid conjugates tested supported mostly callus growth. Simultaneous application of free IAA and its conjugates resulted in the expression of mixed morphogenetic responses (i.e., both vigorous callus growth and organogenesis resulted). Growth kinetics and the effect of temporal exposure of the tissues to the bound and the free auxin suggest that some IAA-amino acid conjugates may specifically influence plant morphogenesis in ways that cannot be easily explained as simply a function of their slow hydrolysis to release free IAA.
Similar content being viewed by others
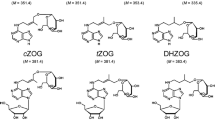
Abbreviations
- IAA:
-
indole-3-acetic acid
- IAA-Ala:
-
N-(indol-3-ylacetyl)-l-alanine
- IAA-Asp:
-
N-(indol-3-ylacetyl)-dl-aspartic acid
- IAA-Lys:
-
N ε-(indol-3-ylacetyl)-l-lysine
- IAA-Orn:
-
N δ-(indol-3-ylacetyl)-l-ornithine
- IAA-Thr:
-
N-(indol-3-ylaцetyl)-l-threonine
References
Bialek K, Meudt W, Cohen JD (1983) Indole-3-acetic acid and IAA conjugates applied to bean stem sections. Plant Physiol 73:130–134
Cohen JD (1982) Identification and quantitative analysis of indole-3-acetyl-l-aspartate from seeds of Glycine max L. Plant Physiol 70:749–753
Cohen JD, Bandurski RS (1982) Chemistry and physiology of the bound auxins. Annu Rev Plant Physiol 33:403–430
Duddeck H, Hiegemann M, Simeonov MF, Kojić-Prodić B, Nigović B, Magnus V (1989) Conformational study of some amino acid conjugates of indol-3-ylacetic acid (IAA) by 1H-NOE-difference spectroscopy. Structure/auxin activity relationships. Z Naturforsch 44c:543–554
Ehmann A (1977) The van Urk-Salkowski reagent—a sensitive and specific reagent for silica gel thin-layer chromatographic detection and identification of indole derivatives. J Chromatogr 132:267–276
Epstein E, Baldi BG, Cohen JD (1986) Identification of indole-3-acetyl-glutamate from seeds of Glycine max L. Plant Physiol 80:256–258
Feung CS, Hamilton RH, Mumma RO (1975) Indole-3-acetic acid. Mass spectra and chromatographic properties of amino acid conjugates. J Agric Food Chem 23:1120–1124
Good NE (1956) The synthesis of indole-3-acetyl-d, l-aspartic acid and related compounds. Can J Chem 34:1356–1358
Hangarter RP, Peterson MD, Good NE (1980) Biological activities of indoleacetyl amino acids and their use as auxins in tissue culture. Plant Physiol 65:761–767
Hangarter RP, Good NE (1981) Evidence that IAA conjugates are slow-release sources of free IAA in plant tissues. Plant Physiol 68:1424–1427
Hutzinger O, Kosuge T (1968) Microbial synthesis and degradation of indole-3-acetic acid. III. The isolation and characterization of indole-3-acetyl-l-lysine. Biochemistry 7:601–605
Kojić-Prodić B, Nigović B, Horvatić D, Ružić-Toroš Ž, Magnus V (1990) Structural studies on conjugates of the plant growth hormone (auxin) with amino acids. XVth Congress of the International Union of Crystallography (Bordeaux, France, July 19–28): Collected Abstracts C-161
Kojić-Prodić B, Nigović B, Horvatić D, Ružić-Toroš Ž, Magnus V, Duax WL, Stezowski JJ, Bresciani-Pahor N (1991) Comparison of the structures of the plant growth hormone indole-3-acetic acid, and six of its amino-acid conjugates. Acta Cryst B47:107–115
Magnus V, Nigović B, Hangarter RP, Good NE (1992) tN-(Indol-3-ylacetyl) amino acids as sources of auxin in plant tissue culture. J Plant Growth Regul (in press)
Mollan RC, Donelly DMX, Harmey MA (1972) Synthesis of indole-3-acetyl aspartic acid. Phytochemistry 11:1485–1488
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Plüss R (1987) Rhizogenese in Grünstecklingen von Populus tremula: Wirkung, Translokation und Metabolismus von exogen applizierter Indolessigsäure. Ph D Thesis, University of Freiburg, Switzerland
Tsurumi S, Wada S (1986) Dioxindole-3-acetic acid conjugates formation from indole-3-acetyl aspartic acid in Vicia seedlings. Plant Cell Physiol 27:1513–1522
Wieland T, Hörlein G (1955) Synthesen einiger β-Indolacetylaminosäuren und -peptide. Justus Liebigs Ann Chem 591:192–199
Wodzicki TJ, Pharis RP, Wodzicki AB (1987) Interaction of indoleacetic acid with its inositol and glucoside conjugates in Avena coleoptile curvature. Plant Physiol 84:1139–1142
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Magnus, V., Hangarter, R.P. & Good, N.E. Interaction of free indole-3-acetic acid and its amino acid conjugates in tomato hypocotyl cultures. J Plant Growth Regul 11, 67–75 (1992). https://doi.org/10.1007/BF00198017
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00198017