Abstract
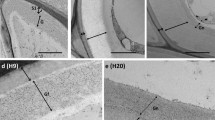
Lectin is the major protein in the phloem tissue of S. japonica. By immunohistochemistry using anti-seed lectin antibody it was demonstrated that the lectin was localized in the ray and the axial parenchyma. Neither lectin nor other cross-reactive materials were observed in the cambium, sieve tubes and companion cells. The distribution and localization changed in relation to tissue development. Lectin content in the bark changed during the year, the average in summer being about 50% of that in winter. The distribution of lectin in the bark in winter was similar from the innermost (youngest) to the outermost (oldest) region. In contrast, in summer the innermost region hardly contained any lectin, and the outermost region contained less lectin than the middle. Lectin localization in tissues and cells differed also depending on tissue age. In new tissue, produced in the current year, lectip was absent in summer, was located in the cytoplasmic layer between cell wall and vacuole in autumn, and sequestered in the vacuoles in winter. On the other hand, lectin in old tissue (formed in the previous year) was located throughout the year mainly within the vacuoles, with only very small contents in the cytoplasmic layer in autumn. Within the outermost (oldest) region, in which the lectin content was low in summer, the cells which bordered the outer bark never contained any lectin in summer. The intracellular localization in autumn in new tissue, determined by immunogold electron microscopy, was in the lumen of the endoplasmic reticulum and vesicles, with gold particles hardly present in the cytoplasm. From these findings we conclude that lectin is synthesized on the endoplasmic reticulum and most vigorously in the new tissue in autumn, and that it is mainly consumed in the outermost bark regions, where dilatation occurs and-or where cork cambium is differentiated.
Similar content being viewed by others
Abbreviations
- ELISA:
-
enzyme-linked immunosorbent assay
- ER:
-
endoplasmic reticulum
- kDa:
-
kilodalton
References
Baba, K., Kuroda, H. (1989) Lectin from the trunk of Sophora japonica. Wood Sci. Technol. 23, 171–178
Baumgartner, B., Tokuyasu, K.T., Chrispeels, M.J. (1980) Immunocytochemical localization in the endoplasmic reticulum of developing bean (Phaseolus vulgaris) cotyledons. Planta 150, 419
Blake, M.S., Johnston, K.H., Russell-Jones, G.J., Gotshlich, E.C. (1984) A rapid, sensitive method for detection of alkaline phosphatase conjugated anti-antibody on western blots. Anal. Biochem. 136, 175–179
Bollini, R., Chrispeels, M.J. (1979) The rough endoplasmic reticulum is the site of reserve-protein synthesis in developing Phaseolus vulgaris cotyledons. Planta 146, 487–501
Bollini, R., Van der Wilden, W., Chrispeels, M.J. (1982) A precursor of the reserve-protein, phaseolin, is transiently associated with the endoplasmic reticulum of developing Phaseolus vulgaris cotyledons. Physiol. Plant. 55, 82–92
Broekaert, W.F., Nsimba-Lubaki, M., Peeters, B., Peumans, W.J. (1984) A lectin from elder (Sambucus nigra) bark. Biochem. J. 221, 163–169
Chrispeels, M.J., Bollini, R. (1982) Characteristics of membrane-bound lectin in developing Phaseolus vulgaris cotyledons. Plant Physiol. 70, 1425–1428
Chrispeels, M.J., Higgins, T.J.V., Craig, S., Spencer, D. (1982) Role of the endoplasmic reticulum in the synthesis of reserve proteins and the kinetics of their transport to protein bodies in developing pea cotyledons. J. Cell Biol. 93, 5–14
Craig, S., Goodchild, D.J. (1984) Periodate-acid treatment of sections permits on-grid immunogold localization of pea seed vicilin in ER and Golgi. Protoplasma 122, 35–44
Esau, K. (1969) Development of phloem. In: The phloem. Encyclopedia of plant anatomy, vol. V, pt. 2, pp. 202–251. Gebrüder Borntraeger, Berlin Stuttgart
Esau, K. (1977) Periderm. In: Anatomy of seed plants, 2nd edn. Wiley, New York
Etzler, M.E. (1986) Distribution and function of plant lectins. In: The lectins, pp. 371–437, Liener, I.E., Sharon, N., Goldstein, I.W., eds. Academic Press, Orlando Fla., USA
Faye, L., Chrispeels, M.J. (1987) Transport and processing of the glycosylated precursor of concanavalin A in jack-bean. Planta 170, 217–224
Gietl, C., Ziegler, H. (1980a) Distribution of carbohydrate-binding proteins in different tissues of Robinia pseudoacacia. Biochem. Physiol. Pflanz. 175, 58–66
Gietl, C., Ziegler, H. (1980b) Affinity chromatography of carbohydrate-binding proteins in the phloem exudate from several tree species. Biochem. Physiol. Pflanz. 175, 50–57
Gietl, C., Kauss, H., Ziegler, H. (1979) Affinity chromatography of a lectin from Robinia pseudoacacia and demonstration of lectins in sieve tube sap from other tree species. Planta 144, 367–371
Goldstein, I.J., Hughes, R.C., Monsigny, M., Osawa, T., Sharon, N. (1980) What should be called a lectin? Nature 285, 66
Greenwood, J.S., Chrispeels, M.J. (1985) Immunocytochemical localization of phaseolin and phytohemagglutinin in the endoplasmic reticulum and Golgi complex of developing bean cotyledons. Planta 164, 295–302
Greenwood, J.S., Stinnisen, H.M., Peumans, W.J., Chrispeels, M.J. (1986) Sambucus nigra agglutinin is located in protein bodies in phloem parenchyma of the bark. Planta 167, 275–278
Hankins, C.N., Kindinger, J.I., Shannon, L.M. (1987) The lectins of Sophora japonica. I. Purification, properties and amino acid sequences of two lectins from leaves. Plant Physiol. 83, 825–829
Hankins, C.N., Kindinger, J.I., Shannon, L.M. (1988) The lectins of Sophora japonica. II. Purification, properties, and N-terminal amino acid sequences of five lectins from bark. Plant Physiol. 86, 67–70
Herman, E.M., Shannon, L.M. (1984) Immunocytochemical localization of concanavalin A in developing jack-bean cotyledons. Planta 161, 94–104
Herman, E.M., Hankins, C.N., Shannon, L.M. (1988) Bark and leaf lectins of Sophora japonica are sequestered in protein-storage vacuole. Plant Physiol. 86, 1027–1031
Hořejši, V., Haškovec, C., Kocourek, J. (1978) Studies on lectins. XXVIII. Isolation and characterization of the lectin from Black locust bark (Robinia pseudoacacia). Biochim. Biophys. Acta 532, 98–104
Hurkman, W.J., Beevers, L. (1982) Sequestration of pea reserve proteins by rough microsomes. Plant Physiol. 69, 1414–1417
Krüpe, M., Ensgraber, A. (1958) Über die Natur der ⋯''Phytoagglutinine” in chemischer, immunochemischer und pflanzenphysiologischer Sicht. Planta 50, 371–378
Laemmli, U.K. (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227, 680–685
Liener, I.E. (1976) Phytohemagglutinins. Annu. Rev. Plant Physiol. 27, 291–319
Nsimba-Lubaki, M., Peumans, W.J. (1986) Seasonal fluctuations of lectins in barks of elderberry (Sambucus nigra) and black locust (Robinia pseudoacacia). Plant Physiol. 80, 747–751
Nsimba-Lubaki, M., Peumans, W.J., Allen, A.K. (1986) Isolation and characterization of glycoprotein lectins from the bark of three species of elder, Sambucus elbus, S. nigra and S. rasemosa. Planta 168, 113–118
Poretz, R.D., Riss, H., Timberlake, J.W., Chien, S. (1974) Purification and properties of hemagglutinin from Sophora japonica seeds. Biochemistry 13, 250–256
Sauter, J.J. (1972) Respiratory and phosphatase activities in contact cells of wood rays and their possible role in sugar secretion. Z. Pflanzenphysiol. 67, 135–145
Towbin, H., Staehlin, T., Gordon J. (1979) Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl. Acad. Sci. USA 76, 4350–4354
Author information
Authors and Affiliations
Additional information
Retired.
Anatomical terms in this paper are used according to “Multilingual glossary of terms used in wood anatomy” edited by the Committee on Nomenclature, International Association of Wood Anatomists; reprints may be obtained from the Office of the Secretary-Treasurer, Universitätsstrasse 2, CH-8092 Zürich 6, Switzerland.
Rights and permissions
About this article
Cite this article
Baba, K., Ogawa, M., Nagano, A. et al. Developmental changes in the bark lectin of Sophora japonica L.. Planta 183, 462–470 (1991). https://doi.org/10.1007/BF00197746
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00197746