Abstract
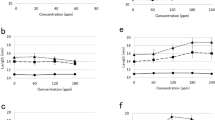
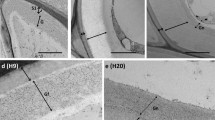
Rapid mobilisation of storage products, including xyloglucan, in cotyledons of germinating nasturtium (Tropaeolum majus L.) normally starts about 7–8 d after imbibition and growth of the seedling at 20–25° C. Levels of activity of endo-1,4-β-glucanase (EC 3.2.1.4) in cotyledons, as assayed viscometrically with xyloglucan as substrate, varied in parallel with the rate of breakdown of xyloglucan. When cotyledons were excised from the seedling axis and incubated on moist filter paper at any point before 7 d, the catabolic reactions which normally occurred in the intact seedling were suspended. If, however, cotyledons excised at 8 d were incubated in 10−6 M 2,4-dichlorophenoxyacetic acid, a rise in endo-1,4-β-glucanase (xyloglucanase) activity was observed and a sharp decrease in fresh and dry weight as well as xyloglucan levels ensued at rates comparable to those observed in cotyledons attached to the seedling. Neither gibberellin nor kinetin treatments promoted xyloglucan breakdown or enhanced xyloglucanase activity. Addition of auxin to excised cotyledons before 7 d did not evoke premature breakdown, indicating that the tissue became receptive to auxin only at this time. The triggering process took place in darkness and was unaffected by various light-dark cycles. It is concluded that the sudden degradation of xyloglucan which occurs in nasturtium seeds about a week after germination begins is the result of enhanced activity of a depolymerizing xyloglucanase, this activity being evoked by auxin originating in the emerging seedling axis.
Similar content being viewed by others
Abbreviations
- 2,4-D:
-
2,4-dichlorophenoxyacetic acid
- 2,3-D:
-
2,3-dichlorophenoxyacetic acid
- GA3 :
-
gibberellic acid
- kDa:
-
kilodalton
References
Akazawa, T., Hara-Nishimura, I. (1985) Topographic aspects of biosynthesis, extracellular secretion and intracellular storage of proteins in plant cells. Annu. Rev. Plant Physiol. 36, 441–472
Bailey, R.W., Bourne, E.J. (1960) Color reactions given by sugars and diphenylamine-aniline spray reagents on paper chromatograms. J. Chromatogr. 4, 206–212
Bewley, J.D., Black, M. (1985) Seeds, physiology of development and germination. Plenum Press, New York
Brummell, D.A., Maclachlan, G.A. (1989) Formation and functions of xyloglucan and derivatives. In: Plant cell wall polymers, pp. 18–32, Lewis, N.G., Paice, M.G., eds. American Chemical Society, Washington, DC
le Dizet, P. (1972) Quelques precisions sur la structure de l'amyloide de capucine. Carbohydr. Res. 24, 505–509
Dubois, M., Gilles, K.A., Hamilton, J.K., Rebas, P.A., Smith, F. (1956) Colorimetric method for determination of sugars and related substances. Anal. Chem. 28, 350–356
Edwards, M., Dea, I.C.M., Bulpin, P.V., Reid, J.S.G. (1985) Xyloglucan (amyloid) mobilisation in the cotyledons of Tropaeolum majus L. seeds following germination. Planta 163, 133–140
Edwards, M., Dea, I.C.M., Bulpin, P.V., Reid, J.S.G. (1986) Purification and properties of a novel xyloglucan-specific endo-(1–4)-β-d-glucanase from germinated nasturtium seeds (Tropaeolum majus L.). J. Biol. Chem. 261, 9489–9494
Edwards, M., Bowman, Y.J.L., Dea, I.C.M., Reid, J.S.G. (1988) A β-d-galactosidase from nasturtium (Tropaeolum majus L.) cotyledons. J. Biol. Chem. 263, 4333–4337
Farkas, V., Maclachlan, G.A. (1988) Stimulation of pea 1,4-β-glucanase activity by oligosaccharides derived from xyloglucan. Carbohydr. Res. 184, 213–219
Halmer, P., Bewley, J.D., Thorpe, T.A. (1976) An enzyme to degrade lettuce endosperm cell walls. Appearance of a mannanase following phytochrome- and gibberellin-induced germination. Planta 130, 189–196
Hayashi, T. (1989) Xyloglucans in the primary cell wall. Annu. Rev. Plant Physiol. Plant Mol. Biol. 40, 139–168
Hayashi, T., Wong, Y.S., Maclachlan, G.A. (1984) Pea xyloglucan and cellulose. II. Hydrolysis by pea endo-1,4-β-glucanases. Plant Physiol. 75, 605–610
Hirasawa, E. (1989) Auxins induce α-amylase activity in pea cotyledons. Plant. Physiol. 91, 484–486
Hoth, A., Blaschek, W., Franz, G. (1986) Xyloglucan (amyloid) formation in the cotyledons of Tropaeolum majus L. seeds. Plant Cell Rep. 5, 9–12
Kooiman, P. (1960) A method for the determination of amyloid in plant seeds. Rec. Trav. Chim. Pays-Bas 79, 675–678
Leung, D.W.M., Bewley, J.D. (1981) Red-light and gibberellic-acid-enhanced α-galactosidase activity in germinating lettuce seeds, cv. Grand Rapids. Planta 152, 436–441
Maclachlan, G.A. (1988) B-glucanases from Pisum sativum. Methods Enzymol. 160, 382–391
Marriott, K.M., Northcote, D.H. (1975) The induction of enzyme activity in the endosperm of germinating castor bean seeds. Biochem. J. 152, 65–70
McDougall, G.J., Fry, S.C. (1988) Inhibition of auxin-stimulated growth of pea stem segments by a specific nonasaccharide of xyloglucan. Planta 175, 412–416
McDougall, G.J., Fry, S.C. (1989) Structure-activity relationships for xyloglucan oligosaccharides with antiauxin activity. Plant. Physiol. 89, 883–887
O'Neill, R.A., White, A.R., York, W.S., Darvill, A.G., Albersheim, P. (1988) A gas chromatographic-mass spectrometric assay for glycosylases. Phytochemistry 27, 329–333
Reid, J.S.G. (1985) Cell wall storage carbohydrates in seeds — biochemistry of the seed “gums” and “hemicelluloses”. Adv. Bot. Res. 11, 125–155
Reid, J.S.G., Bewley, J.D. (1979) A dual role for the endosperm and its galactomannan reserves in the germinative physiology of fenegreek (Trigonella foenum-graecum L.), an endospermic leguminous seed. Planta 147, 145–150
Reid, J.S.G., Meier, H. (1972) The function of the aleurone layer during galactomannan mobilization in germinating seeds of fenugreek (Trigonella foenum-graecum L.), crimson clover (Trifolium incarnatum L.) and lucerne (Medicago sativa L.): a correlative biochemical and ultrastructural study. Planta 106, 44–60
van Onckelen, H.A., Caubergs, R., DeGreef, J. (1977) Effect of light treatment and endogenous growth hormones on α- and β-amylase activity in cotyledons of Phaseolus vulgaris L. Plant Cell Physiol. 18, 1029–1040
Author information
Authors and Affiliations
Additional information
The authors are pleased to acknowledge the technical assistance of Alexander Marcus and valuable discussions with Dr. Vladimir Farkas. This study was supported by a scholarship to A.H. from the Deutsche Forschungsgemeinschaft (FRG) and a grant to G.M. from the Natural Sciences and Engineering Research Council of Canada.
Rights and permissions
About this article
Cite this article
Hensel, A., Brummell, D.A., Hanna, R. et al. Auxin-dependent breakdown of xyloglucan in cotyledons of germinating nasturtium seeds. Planta 183, 321–326 (1991). https://doi.org/10.1007/BF00197728
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00197728