Abstract
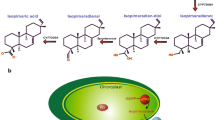
Coproporphyrinogen III oxidase (coprogen oxidase; EC 1.3.3.3) is part of the pathway from 5-aminolevulinate to protoporphyrin IX which is common in all organisms and catalyses oxidative decarboxylation at two tetrapyrrole side chains. We cloned and sequenced fulllength cDNAs encoding coprogen oxidase from barley (Hordeum vulgare L.) and tobacco (Nicotiana tabacum L.). They code for precursor peptides of 43.6 kDa and 44.9 kDa, respectively. Import into pea plastids resulted in a processed tobacco protein of approx. 39 kDa, which accumulated in the stroma fraction. Induction of synthesis of recombinant putative tobacco mature coprogen oxidase consisting of 338 amino-acid residues in Escherichia coli at 20°C result in a catalytically active protein of approx. 39 kDa, while induction of its formation at 37°C immediately terminated bacterial growth, possibly due to toxic effects on the metabolic balance of tetrapyrrole biosynthesis. The plant coprogen oxidase gene was expressed to different extents in all tissues investigated. This is most likely due to the differing requirements for tetrapyrroles in different organs. The steady-state level of mRNA did not significantly differ in etiolated and greening barley leaves. The content of coprogen oxidase RNA reached its maximum in developing cells and decreased drastically when cells were completely differentiated. Functioning of the two photosystems apparatus requires the synthesis of all pigment and protein components during plant development. It is speculated that the enzymes involved in tetrapyrrole synthesis are developmentally rather than light-dependently regulated. Regulation of these enzymes also guarantees a constant flux of metabolic intermediates and avoids photodynamic damage by accumulating porphyrins.
Similar content being viewed by others
Abbreviations
- ALA:
-
5-aminolevulinic acid
- copro:
-
coproporphyrin III
- coprogen:
-
coproporphyrinogen III
- IPTG:
-
isopropyl β-d-thiogalactopyranoside
- LHCP II:
-
light-harvesting chlorophyll-binding protein of photosystem II
- OD:
-
optical density
- proto IX:
-
protoporphyrin IX
- protogen IX:
-
protoporphyrinogen IX
References
Apel K, Kloppstech K (1978) The plastid membrane of barley (Hordeum vulgare). Light induced appearance of mRNA coding for the light-harvesting chlorophyll a/b protein. Eur J Biochem 85: 581–588
Baumgartner BJ, Rapp JC, Mullet JE (1989) Plastid transcription activity and DNA copy number increase early in barley chloroplast development. Plant Physiol 89: 1011–1018
Beale SI, Weinstein JD (1990) Tetrapyrrole metabolism in photosynthetic organisms. In: Dailey HA (ed) Biosynthesis of heme and chlorophyll. McGraw-Hill, New York, pp 287–391
Bogard M, Camadro JM, Nordmann Y, Labbe P (1989) Purification and properties of mouse liver coproporphyrinogen oxidase. Eur J Biochem 181: 417–421
Bonner WM, Laskey RA (1974) A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem 46: 83–88
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254
Camadro J, Chambon H, Jolies J, Labbe P (1986) Purification and properties of Coproporphyrinogen oxidase from the yeast Saccharomyces cerevisiae. Eur J Biochem 156: 579–587
Cavener RD, Ray SC (1991) Eukaryotic start and stop translation sites. Nucleic Acids Res 19: 3185–3193
Chadwick DJ, Ackrill K, eds. (1994) The biosynthesis of the tetrapyrrole pigments. (Ciba Foundation Symposium 180) John Wiley & Sons, Ltd, Chichester
Chomczinsky P, Sacchi N (1987) Single step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 162: 156–159
Elder GH, Evans JO (1978) Evidence that coproporphyrinogen oxidase activity of rat liver is situated in the intermembrane space of mitochondria. Biochem J 172: 345–347
Gavel Y, von Heinje G (1990) A conserved cleavage-site motif in chloroplast transit peptides. FEBS Lett 261: 455–458
Grandchamp B, Phung N, Nordmann Y (1978) The mitochondrial localization of coproporphyrinogen III oxidase. Biochem. J 176: 97–102
Grimm B, Kruse E, Kloppstech K (1989) Transiently expressed early light inducible proteins share transmembrane domains with light-harvesting chlorophyll binding proteins. Plant Mol Biol 13: 583–593
Grossman AR, Bartlett SG, Schmidt GW, Mullet JE, Chua N-H (1982) Optimal conditions for posttranslational uptake of proteins by isolated chloroplasts. J Biol Chem 257: 1558–1563
Hsu WP, Miller GW (1970) Coproporphyrinogen oxidase in tobacco. Biochem J 117: 215–220
Jacobs JM, Jacobs NJ (1993) Porphyrin accumulation and export by isolated barley (Hordeum vulgare) plastids. Plant Physiol. 101: 1181–1187
Kannangara CG, Gough SP (1978) Biosynthesis of Δ-aminolevulinate in greening barley leaves: glutamate 1-semialdehyde aminotransferase. Carlsberg Res Commun 43: 185–194
Kohno H, Furukawa T, Yoshinaga T, Taketani S (1993) Coproporphyrinogen oxidase. Purification, molecular coning and induction of mRNA during erythroid differentiation. J Biol Chem 268: 21359–21363
Kruse E, Kloppstech K (1992) Integration of early light inducible proteins into isolated thylacoid membranes. Eur J Biochem 208: 195–202
Ilag LL, Kumar AM, Söll D (1994) Light regulation of chlorophyll biosynthesis at the level of Δ-aminolevulinate formation in Arabidopsis. Plant Cell 6: 265–275
Madsen O, Sandal L, Sandal NN, Marcker KA (1993) A soybean coproporphyrinogen oxidase gene is highly expressed in root nodules. Plant Mol Biol 23: 35–43
Martasek P, Camadro JM, Delfau-Larue MH, Dumas JB, Montagne JJ, de Verneuil H, Labbe P, Grandchamp B (1994a) Molecular cloning, sequencing, and functional expression of a cDN A encoding human coproporphyrinogen oxidase. Proc Natl Acad Sci USA 91: 3024–3028
Martasek P, Nordmann Y, Grandchamp B (1994b) Homozygous hereditary coproporphyria caused by an arginine to tryptophane substitution in coproporphyrinogen oxidase and common intragenic polymorphisms. Human Mol Gen 3: 477–480
Neville DJ Jr (1971) Molecular weight determination of proteindodecylsulfate complexes by gel-electrophoresis in a discontinuous buffer system. J Biol Chem 246: 6328–6334
Pfisterer J, Lachmann P, Kloppstech K (1982) Transport of proteins into chloroplast. Binding of nuclear-coded chloroplast proteins to the chloroplast envelope J Biochem 126: 143–148
Pötter E, Kloppstech K (1993) Effects of light stress on the expression of early light-inducible proteins in barley. Eur J Biochem 214: 779–786
Roberts BE, Gorecki M, Mulligan RC, Kathleen JD, Rozenblatt S, Rich A (1975) Semian Virus 40 directs synthesis of authentic viral polypeptides in a linked transcription-translation cell-free system. Proc Natl Acad Sci USA 70: 2330–2334
Sambrook J, Fritsch EF, Maniatis T, eds (1989) Molecular cloning: a laboratory manual, 2nd edn, Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
Sanger F, Nicklen S, Coulson AR (1977) DNA sequencing with chain terminating inhibitors. Proc Natl Acad Sci USA 74: 5463–5467
Smith AG, Marsh O, Elder GH (1993) Investigation of the subcellular location of the tetrapyrrole-biosynthesis enzyme coproporphyrinogen oxidase in higher plants. Biochem J 292: 503–508
Smith AG, Griffiths WT (1993) Enzymes of chlorophyll and heme biosynthesis. In: Dey PM, Harborne JB (eds) Methods in plant biochemistry, vol 9, Academic Press Ltd, London, pp 299–344
Stüber D, Ibrahimi I, Cutler D, Dobberstein B, Bujard H (1984) A novel in vitro transcription-translation system: accurate and efficient synthesis of single proteins from cloned DNA sequences. EMBO J 3: 3143–3148
Taketani S, Kohno H, Furukawa T, Yoshinaga T, Tokunaga R (1994) Molecular cloning, sequencing and expression of cDNA encoding human coproporphyrinogen oxidase. Biochim Biophys Acta 1183: 547–549
Troup B, Jahn M, Hungerer C, Jahn D (1994) Isolation of the hemF operon containing the gene for the Escherichia coli aerobic coproporphyrinogen III oxidase by in vivo complementation of a yeast HEM13 mutant. J Bacteriol 176: 673–680
Viro M, Kloppstech K (1980) Differential expression of genes for ribulose-1,5-bisphosphate carboxylase and light-harvesting chlorophyll a/b protein in the developing barley leaf. Planta 105: 41–45
von Heinje G, Steppuhn J, Herrmann RG (1989) Domain structure of mitochondrial and chloroplast targeting peptides. Eur J Biochem 180: 535–545
Xu K, Elliott T (1993) An oxygen-dependent coproporphyrinogen oxidase encoded by the hem F gene of Salmonella typhimurium. J Bacteriol 175: 4990–4999
Yoshinaga T, Sano S (1980a) Coporphyrinogen oxidase I. J Biol Chem 255: 4722–4726
Yoshinaga T, Sano S (1980b) Coporphyrinogen oxidase II. J Biol Chem 255: 4727–4731
Zagorec M, Buhler J-M, Treich I, Keng T, Guarente L, Labbe-Bois R (1988) Isolation, sequence, and regulation by oxygen of the yeast HEM13f gene coding for coproporpohyrinogen oxidase. J Biol Chem 263: 9718–9724
Author information
Authors and Affiliations
Additional information
Accession number: The nucleotide sequence data reported will appear in the EMBL, GenBank and DDBJ Nucleotide Sequence Databases under the accession numbers X82830 (barley coprogen oxidase) and X82831 (tobacco coprogen oxidase).
We are grateful to Prof. Marvin Smith (IPK, Gatersleben, Germany) for his valuable suggestions on earlier versions of the manuscript and to the Ministerium für Wissenschaft und Forschung des Landes Sachsen-Anhalt for financial support to B. Grimm. We thank Petra Linow for skillful technical assistance, Dr. Sakari Kauppinen (Carlsberg Laboratory, Copenhagen, Denmark) for providing the barley cDNA library and Dr. Ole Madsen for providing the soybean coprogen oxidase cDNA clone.
Rights and permissions
About this article
Cite this article
Kruse, E., Mock, HP. & Grimm, B. Coproporphyrinogen III oxidase from barley and tobacco — sequence analysis and initial expression studies. Planta 196, 796–803 (1995). https://doi.org/10.1007/BF00197347
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00197347