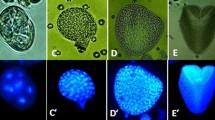
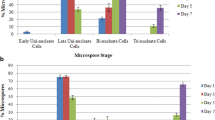
Abstract
Specific stress treatments (sucrose starvation, alone or combined with a heat shock) applied to isolated tobacco (Nicotiana tabacum L.) microspores irreversibly blocked normal gametophytic development and induced the formation of embryogenic cells, which developed subsequently into pollen-derived embryos by culture at 25°C in a sugar-containing medium. A cold shock at 4°C did not inhibit microspore maturation in vitro and did not induce cell division activity, even when combined with a starvation treatment. In the absence of sucrose, microspores isolated in the G1 phase of the cell cycle replicated their DNA and accumulated in G2. Late microspores underwent miotosis during the first day of culture which resulted in a mixed population of bicellular pollen grains and uninucleate microspores, both embryogenic. After the inductive stress treatments the origin of the first multicellular structures, formed in the sugar-containing medium, could be traced to divisions of the microspore cell or divisions of the vegetative cell of bicellular pollen, indicating that the symmetry of microspore mitosis in vitro is not important for embryogenic induction. These results represent a step forward towards a unified model of induction of embryogenesis from microspores/pollen which, within a relatively wide developmental window, are competent to deviate from normal gametophytic development and initiate the alternative sporophytic programme, in response to specific stress signals.
Similar content being viewed by others
Abbreviations
- DAPI:
-
4,6-diamidino-2-phenylindole
References
Bell PR (1992) Apospory and apogamy: implications for understanding the plant life cycle. Int J Plant Sci 153: 123–136
Benito Moreno RM, Macke F, Alwen A, Heberle-Bors E (1988a) In-situ seed production after pollination with in-vitro-matured, isolated pollen. Planta 176: 145–148
Benito Moreno RM, Macke F, Hauser M-T, Alwen A, Heberle-Bors E (1988b) Sporophytes and male gametophytes from in vitro cultured, immature tobacco pollen. In: Cresti M, Gori P, Pacini E (eds) Sexual reproduction in higher plants. Springer, Berlin, pp 137–142
Bensaude O, Mezger V, Morange M (1991) Developmental regulation of heat-shock protein synthesis in unstressed and stressed cells. In: Jeanteur P, Kuchino Y, Müller WEG, Paine PL (eds) Progress in molecular and subcellular biology, vol 12. Springer, Berlin, pp 89–111
Browder LW, Pollock M, Nickells RW, Heikkila JJ, Winning RS (1989) Developmental regulation of the heat-shock response. In: DiBernardino MA, Etkin LD (eds) Genomic adaptability in somatic cell specialization. Developmental biology: a comprehensive synthesis, vol 6. Plenum Press, New York, pp 97–147
Dickinson HG (1994) The regulation of alternation of generation in flowering plants. Biol Rev 69: 419–442
Dworkin M (1979) Spores, cysts, and stalks. In: Sokatch JR, Omston LN (eds) Mechanisms of adaptation. The bacteria, vol VII. Academic Press, New York, pp 1–84
Eady C, Lindsey K, Twell D (1995) The significance of microspore division and division symmetry for vegetative cell-specific transcription and generative cell differentiation. Plant Cell 7: 65–74
Esposito RE, Klapholz S (1981) Meiosis and ascospore development. In: Strathern JN, Jones EW, Broach JR (eds) The molecular biology of the yeast Saccharomyces. Life cycle and inheritance. Cold Spring Harbor Laboratory, New York, pp 211–287
Fan Z, Armstrong KC, Keller WA (1988) Development of microspores in vivo and in vitro in Brassica napus L. Protoplasma 147: 191–199
Garrido D, Charvat B, Benito Moreno RM, Alwen A, Vicente O, Heberle-Bors E (1991) Pollen culture for haploid plant production in tobacco. In: Negrutiu I, Gharti-Chhetri G (eds) A laboratory guide for cellular and molecular plant biology. Birkhäuser, Basel, pp 59–69
Gerisch G (1987) Cyclic AMP and other signals controlling cell development and differentiation in Dictyostelium. Annu Rev Biochem 56: 853–879
Guha S, Maheshwari SC (1964) In vitro production of embryos from anthers of Datura. Nature 204: 497
Guha S, Maheshwari SC (1996) Cell division and differentiation of embryos in the pollen grains of Datura in vitro. Nature 212: 97–98
Heberle-Bors E, Reinert J (1981) Environmental control and evidence for predetermination of pollen embryogenesis in Nicotiana tabacum pollen. Protoplasma 109: 249–255
Heberle-Bors E, Stöger E, Touraev A, Zarsky V, Vicente O (1996) In vitro pollen cultures: progress and perspectives. In: Mohapatra SS, Knox RB (eds) Pollen biotechnology. Gene expression and allergen characterization. Chapman and Hall, New York, pp 85–109
Hoekstra S, van Zijderveld MH, Louwerse JD, Heidekamp F, van der Mark F (1992) Anther and microspore culture of Hordeum vulgare L. cv. Igri. Plant Sci 86: 89–96
Horner M, Street HE (1978) Pollen dimorphism. Origin and significance in pollen plant formation by anther culture. Ann Bot 42: 763–777
Kyo M, Harada H (1986) Control of the developmental pathway of tobacco pollen in vitro. Planta 168: 427–432
Lichter R (1985) From microspores to rape plants: a tentative way to low glucosinolate strains. In: Sorensen H (ed.) Cruciferous crops: production, utilization, description, vol II. Nijhoff/Junk, Dordrecht, pp 268–277
Mejza SJ, Morgant V, DiBona DE, Wong JR (1993) Plant regeneration from isolated microspores of Triticum aestivum. Plant Cell Rep 12: 149–153
Morrison RA, Evans DA (1988) Haploid plants from tissue culture: new plant varieties in a shortened time frame. Biotechnology 6: 684–690
Ogawa T, Fukuoka H, Ohkawa Y (1994) Induction of cell division of isolated pollen grains by sugar starvation in rice. Breed Sci 44: 75–77
Pechan PM, Keller WA (1988) Identification of potentially embryogenic microspores in Brassica napus. Physiol Plant 74: 377–384
Sangwan RS, Sangwan-Norreel BS (1987) Biochemical cytology of pollen embryogenesis. Int Rev Cytol 107: 221–272
Sunderland N, Huang B (1987) Ultrastructural aspects of pollen dimorphism. Int Rev Cytol 107: 175–220
Touraev A, Lezin F, Heberle-Bors E, Vicente O (1995) Maintenance of gametophytic development after symmetrical division in tobacco microspore culture. Sex Plant Reprod 8: 70–76
Touraev A, Ilham A, Vicente O, Heberle-Bors E (1996a) Stressinduced microspore embryogenesis in tobacco: an optimized system for molecular studies. Plant Cell Rep 15: 561–565
Touraev A, Indrianto A, Wratschko I, Vicente O, Heberle-Bors E (1996b) Efficient microspore embryogenesis in wheat (Triticum aestivum L.) induced by starvation at high temperatures. Sex Plant Reprod, in press
Tupý J, Rihová L, Zárský V (1991) Production of fertile tobacco pollen from microspores in suspension culture and its storage for in situ pollination. Sex Plant Reprod 4: 284–287
Vergne P, Delvallée I, Dumas C (1987) Rapid assessment of microspore and pollen development stage in wheat and maize using DAPI and membrane permeabilization. Stain Technol 62: 299–304
Ylstra B, Touraev A, Benito Moreno RM, Stöger E, van Tunen AJ, Vicente O, Mol JNM, Heberle-Bors E (1992) Flavonols stimulate development, germination, and tube growth of tobacco pollen. Plant Physiol 100: 902–907
Zaki MAM, Dickinson HG (1990) Structural changes during the first divisions of embryos resulting from anther and free microspore culture in Brassica napus. Protoplasma 156: 149–162
Zaki MAM, Dickinson HG (1991) Microspore-derived embryos in Brassica: the significance of division symmetry in pollen mitosis I to embryogenic development. Sex Plant Reprod 4: 48–55
Zárský V, Garrido D, Rihová L, Tupý J, Vicente O, Heberle-Bors E (1992) Derepression of the cell cycle by starvation is involved in the induction of tobacco pollen embryogenesis. Sex Plant Reprod 5: 189–194
Zimmerman JL, Cohill PR (1991) Heat shock and thermotolerance in plant and animal embryogenesis. New Biol 3: 641–650
Author information
Authors and Affiliations
Corresponding author
Additional information
We acknowledge the help of Monica Boscaiu and Zarko Hrzenjak with the artwork, and Michaela Braun-Mayer for growing the tobacco plants. This project was financed by the Austrian “Fonds zur Forderung der wissenschaftlichen Forschung”, grant S6003-BIO.
Rights and permissions
About this article
Cite this article
Touraev, A., Pfosser, M., Vicente, O. et al. Stress as the major signal controlling the developmental fate of tobacco microspores: towards a unified model of induction of microspore/pollen embryogenesis. Planta 200, 144–152 (1996). https://doi.org/10.1007/BF00196662
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00196662