Abstract
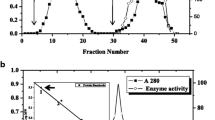
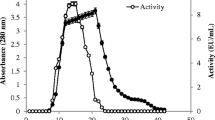
The present study describes the biochemical characteristics of an acid β-fructosidase (EC 3.2.1.26) purified from the fruit of sweet pepper (Capsicum annuum L.). The soluble form, which constitutes more than 95% of the total activity at pH 4.5, hydrolyzes sucrose, raffinose, and stachyose. Its pH and temperature optima are 4.5 and 55 °C, respectively. Metal cations such as Ag+ and Hg2+ strongly inhibit its activity, suggesting the presence of at least one sulfhydryl group at the catalytic site. After purification of the enzyme by means of ammonium sulfate fractionation, gel chromatography (diethyl-aminoethyl-Sephacel, hydroxylapatite, concanavalin A-Sepharose), and preparative gel electrophoresis, the purified enzyme was shown to be a 42 kDa glycoprotein interacting specifically with concanavalin A. After complete chemical deglycosylation with trifluoromethanesulfonic acid, the molecular weight of the constitutive polypeptide was estimated to be 39 kDa. The enzyme glycans were characterized using both affino- and immunodetection. The enzyme has at least two N-linked oligosaccharide sidechains, one of the high-mannose type, and the other of the complex type. The high-mannose glycan has a low molecular weight (1 kDa), and is responsible for the interaction between the enzyme and concanavalin A. The complex-type glycan has an estimated molecular weight of 2 kDa. It contains one β1 → 2-linked xylose residue, probably one fucose residue α 1 → 3-linked to the chitobiose unit, and no terminal galactose residue. The two glycans, associated to the 39 kDa polypeptide, constitute the acid β-fructosidase of the sweet-pepper fruit.
Similar content being viewed by others
Abbreviations
- βF:
-
β-fructosidase
- ConA:
-
concanavalin A
- DEAE:
-
diethylaminoethyl
- DTNB:
-
dithionitrobenzoic acid
- endo F:
-
endo-β-N-acetylglucosamidase F
- endo H:
-
endo-β-N-acetylglucosamidase H
- NEM:
-
N-ethylmaleimide
- PCMB:
-
parachloromercurobenzoate
- PNGase:
-
glycopeptide-N-glycosidase
- TFMS:
-
trifluoromethane sulfonic acid
References
Anderson, R.S., Ewing, E.E., Senesac, A.H. (1980) Inhibition of potato tuber invertase by an endogenous inhibitor. Effects of salts, pH, temperature, and sugars on binding. Plant Physiol. 66, 451–456
Bracho, G.E., Whitaker, J.R. (1990) Purification and partial characterization of potato (Solanum tuberosum) invertase and its endogenous proteinaceous inhibitor. Plant Physiol. 92, 386–394
Bradford, M.M. (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72, 248–254
Chan, H.T., Kwok, S.C.M. (1976) Isolation and characterization of β-fructofuranosidase from papaya. J. Food Sci. 41, 320–323
Chan, H.T., Hibbard, K.L., Goo, T., Akamine, E.K. (1979) Sugar composition of papayas during fruit development. Hort. Sci. 14, 140–141
Davis, B.J. (1964) Disc electrophoresis. II. Method and application to human serum proteins. Ann. NY Acad. Sci. 121, 404–427
Edge, A.S.B., Faltyneck, C.R., Hof, L., Reichert, L.E., Weber, P. (1981) Deglycosylation of glycoproteins by trifluoromethanesulfonic acid. Anal. Biochem. 118, 131–137
Fahrendorf, T., Beck, E. (1990) Cytosolic and cell-wall acid invertases from leaves of Urtica dioica L. a comparison. Planta 180, 237–244
Faye, L. (1981) A new enzymatic staining method for the detection of radish β-fructosidase in gel electrophoresis. Anal. Biochem. 112, 90–95
Faye, L., Chrispeels, M.J. (1985) Characterization of N-linked oligosaccharides by affinoblotting with Concanavalin A-peroxidase and treatment of the blots with glycosidases. Anal. Biochem. 149, 218–224
Faye, L., Chrispeels, M.J. (1988) Common antigenic determinants in the glycoproteins of plants, molluscs and insects. Glycoconjugate J. 5, 245–256
Faye, L., Salier, J.-P. (1989) Crossed affino-immunoelectrophoresis of affino-blotting with lectins: Advantages and limitations for glycoprotein studies. Electrophoresis 10, 841–847
Faye, L., Mouatassim, B., Ghorbel, A. (1986) Cell wall and cytoplasmic isozymes of radish β-fructosidase have different N-linked oligosaccharides. Plant Physiol. 80, 27–33
Frost, G.M., Greenshields, R.N., Tehle, F.W.J. (1968) Some properties of β-fructofuranosidases partially purified from Phaseolus vulgaris and Solanum tuberosum. Biochem. J. 107, 625–636
Hall, A.J. (1977) Assimilate source-sink relationship in Capsicum annuum L. I. The dynamics of growth in fruiting and deflorated plants. Aust. J. Plant Physiol. 4, 623–636
Hubbard, N.L., Pharr, D.M. (1992) Developmental changes in carbohydrate concentration and activities of sucrose metabolizing enzymes in fruits of two Capsicum annuum L. genotypes. Plant Sci. 86, 33–39
Hubbard, N.L., Huber, S.C., Pharr, D.M. (1989) Sucrose phosphate synthase and acid invertase as determinants of sucrose concentration in developing muskmelon (Cucumis melo L.) fruits. Plant Physiol. 91, 1527–1534
Hubbard, N.L., Pharr D.M., Huber, S.C. (1990) The role of sucrose phosphate synthase in sucrose biosynthesis in ripening bananas and its relationship to the respiratory climacteric. Plant Physiol. 94, 201–208
Hubbard, N.L., Pharr, D.M., Huber, S.C. (1991) Sucrose phosphate synthase and other sucrose metabolizing enzymes in fruits of various species. Physiol. Plant. 82, 191–196
Johnson, K.D., Chrispeels, M.J. (1987) Substrate specificities of N-acetylglucosaminyl-, fucosyl-, and xylosyltransferases that modify glycoproteins in the Golgi apparatus of bean cotyledons. Plant Physiol. 84, 1301–1308
Karuppiah, N., Vadlamudi, B., Kaufman, P.B. (1989) Purification and characterization of soluble (cytosolic) and bound (cell wall) isoforms of invertases in barley (Hordeum vulgare) elongating stem tissue. Plant Physiol. 91, 993–998
Kaur, N., Jain, H., Mann, P., Gupta, A.K., Singh, R. (1992) A comparison of properties of invertases and inulase from chicory. Plant Physiol. Biochem. 30, 445–450
Krishnan, H.B., Blanchette, J.T., Okita, T.W. (1985) Wheat invertases: characterization of cell wall bound and soluble forms. Plant Physiol. 78, 241–245
Laemmli, U.K. (1970) Cleavage of structural proteins during assembly of the head of bacteriophage T4. Nature 227, 680–685
Lainé, A.-C., Faye, L. (1988) Significant immunological cross-reactivity of plant glycoproteins. Electrophoresis 9, 841–844
Laurière, C., Laurière, M., Sturm, A., Faye, L., Chrispeels, M.J. (1988) Characterization of β-fructosidase, an extracellular glycoprotein of carrot cells. Biochimie 70, 1483–1491
Laurière, M., Laurière, C., Chrispeels, M.J., Johnson, K.D., Sturm, A. (1989) Characterization of a xylose-specific antiserum that reacts with the complex asparagine-linked glycans of extracellular and vacuolar glycoproteins. Plant Physiol. 90, 1182–1188
Lecari, B. (1982) Changes in invertase activities during the photoperiodically induced bulb formation of onion (Allium cepa). Physiol. Plant. 54, 480–484
Miron, D., Schaffer, A.A. (1991) SPS, SS and invertase activities in developing fruit of Lycopersicon esculentum Mill. and the sucrose accumulating L. hirsutum Humb. and Bonpl. Plant Physiol. 95, 623–627
Nelson, N. (1944) A photometric adaptation of the Somogyi method for the determination of glucose. J. Biol. Chem. 153, 375–380
Nielsen, T.H., Skjaerbaek, H.C., Karlsen, P. (1991) Carbohydrate metabolism during fruit development in sweet pepper (Capsicum annuum) plants. Physiol. Plant. 82, 311–319
Nishizawa, M., Maruyama, Y., Nakamura, M. (1980) Purification and characrization of invertase isozymes from Fusarium oxysporum. Agric. Biol. Chem. 44, 489–498
Ricardo, C.P., ap Rees, T. (1970) Invertase activity during the development of carrot roots. Phytochemistry 9, 239–247
Singh, M.B., Knox, R.B. (1984) Invertases of Lilium pollen. Characterization and activity during in vitro germination. Plant Physiol. 74, 510–515
Sturm, A. (1991) Heterogeneity of the complex N-linked oligosaccharides at specific glycosylation sites of two secreted carrot glycoproteins. Eur. J. Biochem. 199, 169–179
Towbin, H., Staehelin, T., Gordon, J. (1979) Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets. Procedure and some applications. Proc. Natl. Acad. Sci. USA 76, 4350–4354
West, C., Wade, M., McMillan, C., Albersheim, P. (1980) Purification and properties of invertases extractable from Phytophtora megasperma. Arch. Biochem. Biophys. 201, 25–35
Whitaker, J.R. (1963) Determination of molecular weights of proteins by gel filtration on Sephadex. Anal. Chem. 35, 1950–1953
Yelle, S., Chetelat, R.T., Dorais, M., DeVerna, J.W., Bennett, A.B. (1991) Sink metabolism in tomato fruit. IV. Genetic and biochemical analysis of sucrose accumulation. Plant Physiol. 95, 1026–1035
Author information
Authors and Affiliations
Additional information
This work was partly supported by a grant from the Commission Permanente de Coopération Franco-Québécoise to L. Faye, and S. Yelle. D. Michaud was a recipient of a graduate scholarship from the Natural Science and Engineering Research Council of Canada.
Rights and permissions
About this article
Cite this article
Michaud, D., Seye, A., Driouich, A. et al. Purification and partial characterization of an acid β-fructosidase from sweet-pepper (Capsicum annuum L.) fruit. Planta 191, 308–315 (1993). https://doi.org/10.1007/BF00195687
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00195687