Abstract
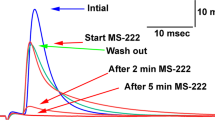
The receptor cells of the ampullary electroreceptor organs of Ictalurus nebulosus bear microvilli on the apical membrane. Whereas microvilli in mechanoreceptive hair cells and in chemoreceptor cells have a transduction function, the function of these membrane specializations in electroreceptor cells is not fully understood. To study the role of the microvilli of the electroreceptor cells, the ampullary electroreceptor organs were apically exposed to the microfilament-disrupting agents cytochalasin B and D. Electrophysiological measurements showed that cytochalasin caused a high decrease in sensitivity and a slight decrease in spontaneous activity. Exposure to cytochalasin B resulted in a striking disorganization of the microvilli on the apical membrane of the electroreceptor cells. The most plausible explanation for the results is that treatment with cytochalasin mainly affects the actin filaments of the microvilli causing an increase of the resistance of the apical membrane. A high apical resistance results in a decrease of the voltage over the basal membrane, which in turn reduces the sensitivity. The conclusion is that intact apical microvilli are necessary for proper functioning of ampullary electroreceptor organs. Alterations in microvillar properties, like surface area and ion channel conductancy might play a considerable role in the regulation of the sensitivity.
Similar content being viewed by others
References
Akoev GN, Andrianov GN (1989) Synaptic transmission in mechano- and electroreceptors of the acousticolateral system. Progr Sensory Physiol 9:53–95
Andrianov GN, Bretschneider F, Peters RC, Teunis PMF (1992) In vitro electroreceptor organs for pharmacological studies. J Neurosci Meth 44:1–6
Anholt RRH (1991) Odor recognition and olfactory transduction: the new frontier. Chem Senses 16(5):421–427
Ashmore JF (1991) The electrophysiology of hair cells. Annu Rev Physiol 53:465–476
Bennett MVL (1971) Electroreception. In: Hoar WS, Randall DS (eds) Fish physiology. Academic Press, New York, pp493–574
Bennett MVL, Clusin WT (1979) Transduction at electroreceptors: origins of sensitivity. In: Cone RA, Dowling JE (eds) Membrane transduction mechanisms. Raven Press, New York,pp 91–116
Bennett MVL, Obara S (1986) Ionic mechanism and pharmacology of electroreceptors. In: Bullock TH, Heiligenberg W (eds) Electroreception. John Wiley and Sons, New York, pp 157–181
Bershadsky AD, Vasiliev JM (1988) Systems of actin filaments. In: Bershadsky AD, Vasiliev (eds) Cytoskeleton. Plenum Press, New York, London, pp 13–78
Bretschneider F, Peters RC (1992) Transduction and transmission in ampullary electroreceptors of catfish. Comp Biochem Physiol 103A(2):245–252
Bretschneider F, Peters RC, Peele PH, Dorresteijn AW (1980) Functioning of catfish electroreceptors: statistical distribution of sensitivity and fluctuations of spontaneous activity. J Comp Physiol 137A:273–279
Burgess DR, Grey RD (1974) Alterations in morphology of developing microvilli elicted by cytochalasin B. J Cell Biol 62:566–574
Cappuccinelli P (1980) The motility system of eukaryotic cells. In: Cappucinelli P (ed) Motility of living cells. Chapman and Hall Ltd, New York, pp 24–58
Cheek TR, Burgoyne RD (1991) Cytoskeleton in secretion and neurotransmitter release. In: Burgoyne RD (ed) The neuronal cytoskeleton. Wiley-Liss, New York, pp 309–325
Clauss W, Dantzer V, Skadhauge E (1988) A low salt diet facilitates Cl secretion in hen lower intestine. J Membr Biol 102:83–96
Cooper JA (1987) Effects of cytochalasin and phalloidin on actin. J Cell Biol 105:1473–1478
Fields RD, Ellisman MH (1985) Synaptic morphology and differences in sensitivity. Science 228:197–199
Hirokawa N (1991) Molecular architecture and dynamics of the neuronal cytoskeleton. In: Burgoyne RD (ed) The neuronal cytoskeleton. Wiley-Liss, New York, pp 5–74
Jørgensen JM (1989) Evolution of octavolateralis sensory cells. In: Coombs S, Görner P, Münz H (eds) The mechanosensory lateral line. Neurobiology and evolution. Springer New York, pp115–145
Jørgensen JM (1992) The electrosensory cells of the ampullary organ of the transparent catfish (Kryptopterus bicirrhus). Acta Zool Stockholm 73(2):79–83
Jørgensen JM, Bullock TH (1987) Organization of the ampullary organs of the African knife fish Xenomystus nigri (Teleostii: Notopteridae). J Neurocytol 16:311–315
Kinnamon SC (1988) Taste transduction: a diversity of mechanisms. Trends Neurosci 11(11):491–496
Lewis SA, de Moura JLC (1984) Apical membrane area of rabbit urinary bladder increases by fusion of intracellular vesicles: an electrophysiological study. J Membr Biol 82:123–136
MacLean-Fletcher S, Pollard TD (1980) Mechanism of action of cytochalasin B on actin. Cell 20:329–341
Mullinger AM (1964) The fine structure of ampullary electric receptors in Amiurus. Proc R Soc (Lond) B 160:345–359
Nilsson M, Mölne J, Ericson LE (1991) Integrity of the occluding barrier in high-resistant thyroid follicular epithelium in culture. II. Immediate protective effect of TSH on paracellular leakage induced by Ca2+ removal and cytochalasin B. Eur J Cell Biol 56:308–318
Peters RC, Teunis PFM, Bretschneider F, van Weerden R (1988) Ampullary electroreceptors in neurophysiological instruction. J Biol Educ 22(1):61–66
Peters RC, Zwart R, Loos WJG, Bretschneider F (1989) Transduction at electroreceptor cells manipulated by exposure of apical membranes to ionic channel blockers. Comp Biochem Physiol 94C(2):663–669
Pickles JO, Corey DP (1992) Mechanoelectrical transduction by hair cells. Trends Neurosci 15(7):254–259
Ravdin JI, Guerrant RL, Sperelakis N (1985) Entamoeba histolytica: Impedance measurements and cytotoxicity in the presence of bepredil, verapamil, and cytochalasin D. Exp Parasitol 60:63–72
Stitt AW, Fairweather I (1991) Fasciola hepatica: the effect of the microfilament inhibitor cytochalasin B on the ultrastructure of the adult fluke. Parasitol Res 77:675–685
Sugawara Y (1989) Electrogenic Na-K pump at the basal face of the sensory epithelium in the Plotosus electroreceptor. J Comp Physiol A 164:598–599
Teunis PFM, Bretschneider F, Peters RC (1990) Denervation changes the transmission properties of electroreceptor sensory synapses. Comp Biochem Physiol 94A:647–651
Trifaró JM, Vitale ML, Roderíguez Del Castillo A (1992) Cytoskeleton and molecular mechanism in neurotransmitter release by neurosecretory cells. Eur J Pharmacol Molec Pharmacol Sec 225: 83–104
Turnheim K (1991) Intrinsic regulation of apical sodium entry in epithelia. Physiol Rev 71(2):429–445
Urbanik E, Ware BR (1989) Actin filament capping and cleaving activity of cytochalasins B, D, E, and H. Arch Biochem Biophys 269(1):181–187
Wachtel A, Szamier RB (1969) Special cutaneous receptor organs of fish. IV. Ampullary organs of the nonelectric catfish, Kryptopterus. J Morphol 128: 291–302
Watson GM, Hessinger DA (1991) Chemoreceptor-mediated elongation of stereocilium bundles tunes vibration-sensitive mechanoreceptors on cnidocyte-supporting cell complexes to lower requencies. J Cell Sci 99:307–316
Zakon HH (1986) The electroreceptive periphery. In: Bullock TH, Heiligenberg W (eds) Electroreception. John Wiley and Sons, New York, pp 103–156
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Heijmen, P.S., Peters, R.C. Apically administered cytochalasin B and D decreases sensitivity of electroreceptor organs in the North-American catfish, Ictalurus nebulosus . J Comp Physiol A 175, 279–287 (1994). https://doi.org/10.1007/BF00192987
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00192987