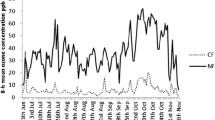
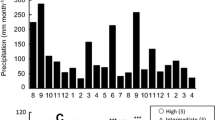
Abstract
Scots pine (Pinus sylvestris L.) seedlings were fumigated with 1.2–1.5 x ambient ozone (cumulative exposure) over 2 seasons in an open-air experiment. Starch and fatty acid concentrations were analyzed in needle and root tissue in the summer, autumn and early winter. Seedling growth was determined by measuring the height of the stem and the total shoot and root biomass. Significant decreases in growth were found in exposed seedlings, even though visible symptoms were lacking. Almost significant reductions in needle and root starch concentrations were found. In the ozone treated foliage, significant increases in myristic acid (14∶0) were detected, but the major fatty acids remained unchanged. Fatty acid ratios showed that the degree of unsaturation decreased in treated needles in the summer. In the roots of ozone treated seedlings, changes in fatty acids were different from those in the foliage. Decreases of the main root fatty acids (16∶0, 18∶0, 18∶1, 18:2, 18∶3) were detected in the summer. These results show that Scots pine is susceptible to enhanced levels of ozone. If the tropospheric ozone levels continue to increase it may have deleterious effects on Scots pine forests in Finland.
Similar content being viewed by others
References
Andersen CP, Hogsett WE, Wessling R, Plocher M (1991) Ozone decreases spring root growth and root carbohydrate content in ponderosa pine the year following exposure. Can J For Res 21: 1288–1291
Anttonen S, Herranen J, Peura P, Kärenlampi L (1995) Fatty acids and ultrastructure of ozone-exposed Aleppo pine (Pinus halepensis Mill.) needles. Environ Pollut 87: 235–242
Barnes JD, Davison AW (1988) The influence of ozone on the winter hardiness of Norway spruce [Picea abies (L.) Karst]. New Phytol 108: 159–166
Carlsson A, Sandelius A-S, Sellden G (1990) Exposure of Norway spruce [Picea abies (L.) Karst.] to ozone in open-top chambers. Effects on membrane lipid acyl composition in needles during the winter season. Physiol Plant 79: A124
Cassagne C, Lessire R, Bessoule JJ, Moreau P, Creach A, Schneider F, Sturbois B (1994) Biosynthesis of very long chain fatty acids in higher plants. Prog Lipid Res 33: 55–69
Chappelka AH, Chevone BI (1986) White ash seedling growth response to ozone and simulated acid rain. Can J For Res 16: 786–790
Chappelka AH, Chevone BI (1992) Tree responses to ozone. In: Lefohn A (eds) Surface level ozone exposures and their effects on vegetation. Lewis, Chelsea, Mich. pp 271–324
Chappelka AH, Freer-Smith PH (1995) Prodisposition of trees by air pollutants to low temperatures and moisture stress. Environ Pollut 87: 105–117
Cooley DR, Manning WJ (1987) The impacts of ozone on assimilate partitioning in plants: a review. Environ Pollut 47: 95–113
Cooper KM, Lösel DM (1978) Lipid physiology of vesicular arbuscular mycorrhiza. I. Composition of lipids in roots of onion, clover and ryegrass infected with Glomus mosseae. New Phytol 80: 143–151
Criegee R (1975) Mechanisms of ozonolysis. Angew Chem 14: 745–760
Darrall NM (1989) The effect of air pollutants on physiological processes in plants. Plant Cell Environ 12: 1–30
Davis DD, Wood FA (1972) The relative susceptibility of eighteen coniferous species to ozone. Phytopathology 62: 14–19
Fangmeier A, Kress LW, Lepper P, Heck WW (1990) Ozone effects on the fatty acid composition of loblolly pine (Pinus taeda L.). New Phytol 115: 639–647
Fincher J, Cumming JR, Alscher RG, Rubin G, Weinstein L (1989) Long-term ozone exposure affects winter hardiness of red spruce (Picea rubens Sarg.). New Phytol 113: 85–96
Friend AL, Tomlinson PT (1992) Mild ozone exposure alters 14C dynamics in foliage of Pinus taeda L. Tree Physiol 11: 215–227
Friend AL, Tomlinson PT, Dickson RE, O'Neill EG, Edwards NT, Taylor GE Jr (1992) Biochemical composition of loblolly pine reflects pollutant exposure. Tree Physiol 11: 35–47
Gorissen A, Joosten NN, Jansen AE (1991a) Effects of ozone and ammonium sulphate on carbon partitioning to mycorrhizal roots of juvenile Douglas fir. New Phytol 119: 243–250
Gorissen A, Schelling GC, Veen JA van (1991b) Concentration dependent effects of ozone on translocation of assimilates in Douglas fir. J Environ Qual 20: 169–173
Grunwald C, Endress AG (1985) Foliar sterols in soybeans exposed to chronic levels of ozone. Plant Physiol 77: 245–247
Gueth S, Frenzel B (1989) Epicuticularwachs der Tanne (Abies alba Mill.) und Walderkrankung. II Zusammensetzung der Cuticularwachse und Nadellipide. Angew Bot 63: 259–277
Halliwell B, Gutteridge JMC (1989) Free radicals in biology and medicine. Clarendon, Oxford
Heath RL (1984) Air pollutant effects on biochemicals derived from metabolism: organic, fatty and amino acids. In: Koziol MJ, Whatley FR (eds) Gaseous air pollutants and plant metabolism. Butterworths, London, pp 275–290
Heath RL (1987) The biochemistry of ozone attack on the plasma membrane of plant cells. In: Saunders JA, Kosak-Channing L, Conn EE (eds) Recent advances in phytochemistry phytochemical effects of environmental compounds, vol 21. Plenum Press, New York, pp 29–54
Heath RI (1994) Alterations of plant metabolism by ozone exposure. In: Alcher RG, Wellburn AR (eds) Plant responses to the gaseous environment. Chapman and Hall, London, pp 121–145
Heath RL, Castillo FJ (1988) Membrane disturbances in response to air pollutants. In: Schulte-Hostede N, Darrall LW, Wellburn AR (eds) Air pollution and plant metabolism, Elsevier, London, pp 55–75
Holopainen T, Rantanen L (1992) Effect of open field ozone fumigation on the development of Scots pine and Norway spruce mycorrhizas. In: Air pollution and interactions betwen organisms in forest ecosystems. Proc of IUFRO Meeting, Tharandt/Dresden, Germany, 9–11 September 1992, pp 223–227
Jamieson GR, Reid EH (1972) The leaf lipids of some conifer species. Phytochemistry 11: 269–275
Jensen KF (1981) Ozone fumigation decreases the root carbohydrate content and dry weight of green ash seedlings. Environ Pollut 26: 147–152
Kainulainen P, Holopainen JK, Hyttinen H, Oksanen J (1994) Effect of ozone on the biochemistry and aphid infestation of Scots pine. Phytochemistry 35: 39–42
Kickert RN, Krupa SV (1990) Forest responses to tropospheric ozone and global climate change: an analysis. Environ Pollut 68: 29–65
Kress LW, Skelly JM (1982) Response of several eastern forest tree species to chronic doses of ozone and nitrogen dioxide. Plant Disease 66: 1149–1152
Kyburz S, Eichenberger W, Keller T, Rennenberg H, Schröder P (1991) Fatty acids of ozone exposed Scots pine and poplar trees and of damaged field-grown Norway spruce trees. Eur J For Pathol 21: 49–56
Laisk A, Kull O, Moldau H (1989) Ozone concentration in leaf intercellular air spaces is close to zero. Plant Physiol 90: 1163–1167
Laurila T, Hakola H, Joffre SM, Lättilä H, Koskinen T (1994) Tropospheric chemistry of greenhouse gases in Finnish conditions. In: Kanninen M, Heikinheimo P (eds) The Finnish research programme on climate change, second progress report. Publications of the Academy of Finland 1/1994, VAPK-Publishing, Helsinki, pp 85–90
Lefohn AS (1992) The characterization of ambient ozone exposures. In: Lefohn A (eds) Surface level ozone exposures and their effects on vegetation. Lewis, Chelsea, Mich, pp 31–92
Liljenberg C, Karunen P, Ekman R (1984) Water stress induced changes in steryl lipids of oat root cells. In: Siegenthaler P-A, Eichenberger W (eds) Developments in plant biology. 9. Structure, function and metabolism of plant lipids, Elsevier, Amsterdam, pp 613–617
Luethy-Krause B, Landolt W (1990) Effects of ozone on starch accumulation in Norway spruce (Picea abies). Trees 4: 107–110
Mackay CE, Cenaratna T, McKersie BD, Fletcher RA (1987) Ozone induced injury to cellular membranes in Triticum aestivum L. and protection by triazole S-3307. Plant Cell Physiol 28: 1271–1278
Meier S, Grand LF, Schoneberger MM, Reinert RA, Bruck RI (1990) Growth, ectomycorrhizae and nonstructural carbohydrates of loblolly pine seedlings exposed to ozone and soil water deficit. Environ Pollut 64: 11–27
Murphy DJ (1994) Biogenesis, structure and biotechnology of plant storage lipids. Prog Lipid Res 33: 71–85
Nouchi H, Toyama S (1988) Effects of O3 and peroxyacetylnitrate on polar lipids and fatty acids in leaves of morning glory and kidney bean. Plant Physiol 87: 638–646
Pääkkonen E, Paasisalo S, Holopainen T, Kärenlampi L (1993) Growth and stomatal responses of birch (Betula pendula Roth.) clones to ozone in open-air and chamber fumigations. New Phytol 125: 615–623
Pauls KP, Thompson JE (1981) Effects of in vitro treatment with ozone on the physical and chemical properties of membranes. Physiol Plant 53: 255–262
Pell EJ, Eckardt NA, Glick RE (1994) Biochemical and molecular basis for impairment of photosynthetical potential. Photosynth Res 39: 453–462
Prinz B, Krause GHM, Jung KD (1987) Development of novel forest decline in Germany. In: Hutchinson TC, Meema KM (eds) Effects of atmospheric pollutants on forests, wetlands and agricultural ecosystems, Springer, Berlin Heidelberg New York, pp 1–24
Pye JM (1988) Impact of ozone on growth and yield of trees: a review. J Environ Qual 17: 347–360
Reich PB, Amundson RG (1985) Ambient levels of ozone reduce net photosynthesis in tree and crop species. Science 230: 566–570
Reich PB, Lassoie JP (1984) Effects of low level O3 exposure on leaf diffusive conductance and water-use efficiency in hybrid poplar. Plant Cell Environ 7: 661–668
Reich PB, Schoettle AW, Stroo HF, Troiano J, Amundson RG (1985) Effects of O3, SO2 and acidic rain on mycorrhizal infection in northern red oak seedlings. Can J Bot 63: 2049–2055
Runeckles VC, Chevone BI (1992) Crop responses to ozone. In: Lefohn A (ed) Surface level ozone exposures and their effects on vegetation. Lewis, Chelsea, Mich. pp 189–270
Runeckles VC, Krupa SV (1994) The impact of UV-B radiation and ozone on terrestrial vegetation. Environ Pollut 83: 191–213
Sakaki T, Kondo N, Sugahara K (1983) Breakdown of photosynthetic pigments and lipids in spinach leaves with ozone fumigation: role of active oxygens. Physiol Plant 59: 28–34
Sakaki T, Ohnishi J, Kondo N, Yamada M (1985) Polar and neutral lipid changes in spinach leaves with ozone fumigation: triacylglycerol synthesis from polar lipids. Plant Cell Physiol 26: 2253–2262
Sakaki T, Kondo N, Yamada M (1990) Pathway for the synthesis of triacylglycerols from monogalactocyldiacylglycerols in ozone-fumigated spinach leaves. Plant Physiol 94: 773–780
Sakaki T, Tanaka K, Yamada M (1994) General metabolic changes in leaf lipids in response to ozone. Plant Cell Physiol 35: 53–62
SAS Institute (1988) SAS Procedures guide, 6.03 edition. SAS Institute, Cary, N. C.
Sasek TW, Richardson CJ, Fendick EA, Bevington SR, Kress LW (1991) Carryover effects of acid rain and ozone on the physiology of multiple flushes of loblolly pine seedlings. For Sci 4: 1078 -1098
Selstam E, Öquist G (1985) Effects of frost hardening on the composition of galactolipids and phospholipids occuring during the isolation of chloroplasts thylakoids from needles of Scots pine. Plant Sci 42: 41–48
Spence RD, Rykiel EJ, Sharpe PJH (1990) Ozone alters carbon allocation in loblolly pine: assessment with carbon-11 labeling. Environ Pollut 64: 93–106
Tingey DT, Wilhourn RG, Standley C (1976) The effect of chronic ozone exposures on the metabolite content of ponderosa pine seedlings. For Sci 22: 234–241
Wallin G, Skärby L (1992) The influence of ozone on the stomatal and non-stomatal limitation of photosynthesis in Norway spruce, Picea abies (L.) Karst, exposed to soil moisture deficit. Trees 6: 128–136
Wallin G, Skärby L, Sellden G (1990) Long term exposure of Norway spruce Picea abies (L.) Karst, to ozone in open top chambers. I. Effects on the capacity of net photosynthesis, dark respiration and leaf conductance of shoots of different ages. New Phytol 115: 335–344
Wellburn AR, Robinson DC, Thomson A, Leith I (1994) Influence of episodes of summer O3 on Δ5 and Δ9 fatty acids in autumnal lipids of Norway spruce [Picea abies (L.) Karst]. New Phytol 127: 355–361
Wingsle G, Mattson A, Ekblad A, Hällgren J-E, Selstam E (1992) Activities of glutathione reductase and Superoxide dismutase in relation to changes of lipids and pigments due to ozone in seedlings of Pinus sylvestris L. Plant Sci 82: 167–178
Wolfenden J, Wellburn AR (1991) Effects of summer ozone on membrane lipid composition during subsequent frost hardening in Norway spruce [Picea abies (L.) Karst]. New Phytol 118: 323–329
Wulff A, Hänninen O, Tuomainen A, Kärenlampi L (1992) A method for open-air exposure of plants to ozone. Ann Bot Fenn 29: 253–262
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Anttonen, S., Kärenlampi, L. Fatty acids, starch and biomass of Scots pine needles and roots in open-air ozone exposure. Trees 10, 74–82 (1995). https://doi.org/10.1007/BF00192186
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00192186