Abstract
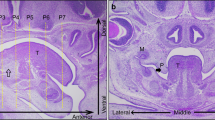
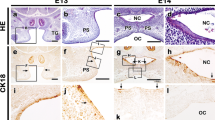
The sequence of events and the distribution of extracellular matrix (ECM) components was examined during mouse secondary palatal shelf elevation in an in vitro model using standard roller tube culture methods developed for the culture of early embryos. In this culture system, the morphological changes associated with remodelling and reorientation of the palatal shelves of gestational day 13 mouse fetuses were similar to those observed in vivo. However, in specimens explanted 24–30 h prior to reorientation in vivo, remodelling began rapidly after explantation, and significant reorientation was accomplished within 4 h. Midline contact between the shelves did not occur until after 18 h in vitro, concomitant with shelf growth. Therefore, in this in vitro model, events related to palatal shelf remodelling and reorientation can be distinguished from those related to shelf growth. We used this in vitro model to characterize the transient changes in ECM distribution and accumulation that occur concomitant with events in shelf remodelling. Our results show that, during rapid remodelling in vitro, the relative distributions of collagen III, fibronectin and hyaluronate, as visualized by immunofluorescent staining, decreased within specific regions of the mesenchymal compartment. In contrast, the distribution of collagen I within the mesenchyme increased, and the distribution of tenascin did not change significantly. All molecules examined, except tenascin, showed changes in distribution within the basement membrane. These patterns of distribution are similar to those observed during more gradual remodelling in vivo. During remodelling in vitro, the deposition of [3H]-glucosamine- and [3H]-proline-labelled components of the ECM, as visualized by autoradiography, was greatest during the first 3 h of culture. During this period, labelled ECM accumulated within specific regions of the mesenchyme and palatal epithelial basement membrane. Uptake was reduced dramatically during the subsequent 3 h in culture and was restricted mainly to the palatal epithelium and its underlying basement membrane. The in vitro system permitted the characterization of early events in shelf remodelling leading to reorientation. Results suggest that remodelling is accompanied by rapid, local accumulation of ECM in specific regions of the palatal shelf previously thought to play a role in the process.
Similar content being viewed by others
References
Brinkley L (1980) In vitro studies of palatal shelf elevation. In: Pratt R, Christiansen R (eds) Current research trends in prenatal craniofacial development. Elsevier North Holland, New York, pp 204–220
Brinkley L (1984) Changes in cell distribution during mouse secondary palate closure in vivo and in vitro. I. Epithelial cells, Dev Biol 102:216–227
Brinkley L, Morris-Wiman J (1987) Computer-assisted analysis of hyaluronate distribution during morphogenesis of the mouse secondary palate. Development 100:629–635
Brinkley L, Vickerman M (1982) The effects of chlorcyclizine-induced alterations of glycosaminoglycans on mouse palatal shelf elevation in vivo and in vitro. J Embryol Exp Morphol 69:193–213
Brinkley L, Basehoar B, Branch A, Avery J (1975) A new in vitro system for studying secondary palatal development. J Embryol Exp Morhol 34:485–495
Diewert V (1974) A cephalometric study of orofacial structures during secondary palate closure in the rat. Arch Oral Biol 19:303–315
Diewert V, Tait B (1979) Palatal process movement in the rat as demonstrated in frozen sections. J Anat 128:609–618
Ferguson M (1988) Palate development. Development 103 [Suppl]:41–69
Foreman D, Sharpe P, Ferguson M (1991) Comparative biochemistry of mouse and chick secondary palate development in vivo and in vitro with particular emphasis on extracellular matrix molecules and the effects of growth factors on their synthesis. Arch Oral Biol 36:457–471
Green S, Tarone G, Underbill C (1988) Distribution of HA and HA-receptors in the adult lung. J Cell Sci 89:145–156
Knudsen T, Bulleit R, Zimmerman E (1988) Histochemical localization of glycosaminoglycans during morphogenesis of the secondary palate in mice. Anat Embryol 173:137–142
Eewis C, Thibault L, Pratt R, Brinkley E (1980) An improved culture system for secondary palate elevation. In Vitro 16:453–460
Morris-Wiman J, Brinkley L (1992) An extracellular matrix infrastructure provides support for murine secondary palatal shelf remodelling. Anat Rec 234:575–586
New D, Coppola P, Terry S (1973) Culture of explanted rat embryos in rotating tubes. J Reprod Fertil 35:1135–1138
Pratt R, King C (1971) Collagen synthesis in the secondary palate of the developing rat. Arch Oral Biol 16:1181–1185
Pratt R, Goggins J, Wilk A, King C (1973) Acid mucopolysaccharide synthesis in the secondary palate of the developing rat at the time of rotation and fusion. Dev Biol 32:230–237
Sharpe P, Ferguson M (1988) Mesenchymal influences of epithelial differentiation in developing systems. J Cell Sci [Suppl-10]:195–230
Silver M, Foidart J-M, Pratt R (1981) Distribution of fibronectin and collagen during mouse limb and palate development. Differentiation 18:141–149
Smiley G, Koch W (1972) An in vitro and in vivo study of single palatal processes. Anat Rec 73:405–416
Toole B (1981) Glycosaminoglycans in morphogenesis. In: Hay E (ed) Cell biology of the extracellular matrix. Plenum Press. New York, pp 259–294
Walker B (1969) Correlation of embryonic movement with palate closure in mice. Teratology 2:191–198
Walker B, Crain F (1960) Effects of hypervitaminosis A on palate development in two strains of mice. Am J Anat 107:49–58
Walker B, Fraser C (1956) Closure of the secondary palate in three strains of mice. J Embryol Exp Morphol 4:176–189
Walker B, Quarles J (1976) Palate development in mouse foetuses after tongue removal. Arch Oral Biol 21:405–412
Wilk A, King C, Pratt R (1978) Chlorcyclizine induction of cleft palate in the rat: degradation of palatal glycosaminoglycans. Teratology 28:199–210
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Morris-Wiman, J., Brinkley, L. Rapid changes in the extracellular matrix accompany in vitro palatal shelf remodelling. Anat Embryol 188, 75–85 (1993). https://doi.org/10.1007/BF00191453
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00191453