Summary
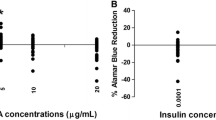
Ribose has been used successfully in the treatment of ischemic heart disease and muscular enzyme deficiencies, and its administration also facilitates the diagnosis of coronary artery disease by influencing thallium-201 scintigraphy. Concerns about the safety of ribose therapy have been triggered by reports about inhibitory effects of ribose on cell proliferation in vitro. This study examines possible side effects of ribose on human lymphocytes. Unstimulated and mitogen-stimulated human lymphocytes were incubated with ribose concentrations associated with high-dose oral administration, i.e., 3.5 mM, and with two- (7 mM) and tenfold (35 mM) higher concentrations. Cell cultures with matching glucose concentrations served as controls. Incorporation of [3H]thymidine into cells was used to measure cell proliferation. No significant inhibition of human lymphocyte proliferation in vitro was observed in mitogen-stimulated cells. Unstimulated cultures showed significant inhibition only at 35 mM ribose. It is concluded that ribose plasma levels associated with high-dose oral administration do not inhibit human lymphocyte proliferation in vitro. No evidence was found that short-term ribose therapy is harmful to human lymphocytes.
Similar content being viewed by others
References
Gross M, Reiter S, Zöllner N (1989) Metabolism of d-ribose administered continuously to healthy persons and to patients with myoadenylate deaminase deficiency. Klin Wochenschr 67:1205–1213
Gross M, Zöllner N (1991) Serum levels of glucose, insulin, and C-peptide during long-term d-ribose administration in man. Klin Wochenschr 69:31–36
Hegewald MG, Palac RT, Angello DA, Perlmutter NS, Wilson RA (1991) Ribose infusion accelerates thallium redistribution with early imaging compared with late 24-hour imaging without ribose. J Am Coll Cardiol 18:1671–1681
Kornbluth J, Raab SS, Wilson DB (1984) Inhibition of cell-mediated lympholysis by cloned and uncloned lines of natural killer cells and cytotoxic T lymphocytes with sugars and lecitins. Cell Immunol 88:182–173
MacDermot RP, Kienker LJ, Bertovich MJ, Muchmore AV (1981) Inhibition of spontaneous but not antibody-dependent cell-mediated cytotoxicity by simple sugars: evidence that endogenous lecitins may mediate spontaneous cell-mediated cytotoxicity. Immunology 44:143–152
Marini M, Zunica G, Francheschi C (1985) Inhibition of cell proliferation by d-ribose and deoxy-d-ribose. Proc Soc Exp Biol Med 180:246–257
Mauser M, Hoffmeister HM, Nienaber C, Schaper W (1985) Influence of ribose, adenosine, and “AICAR” on the rate of myocardial adenosine triphosphate synthesis during reperfusion after coronary artery occlusion in the dog. Circ Res 56:220–230
Perlmutter NS, Wilson RA, Angello DA, Palac RT, Lin J, Brown BG (1991) Ribose facilitates thallium-201 redistribution in patients with coronary artery disease. J Nucl Med 32:193–200
Pliml W, von Arnim T, Stäblein A, Hofmann H, Zimmer H-G, Erdmann E (1992) Effects of ribose on exercise-induced ischaemia in stable coronary artery disease. Lancet 340:507–510
Reimers CD, Pongratz DE, Gross M, Paetzke I, Zimmer H-G (1988) Symptomatische Therapie des primären Myoadenylat-Deaminase-Mangels sowie der Glykogenose Typ V mit d-Ribose. In: Mortier W, Pothmann R, Kunze K (eds) Aktuelle Aspekte neuromuskulärer Erkrankungen. Therapie, Früherkennung, Genetik, Mitochondriopathien. Thieme, Stuttgart New York, pp 127–130
Stankova J, Rola-Pleszczynski M (1984) α-Fucose inhibits human mixed-lymphocyte culture reactions and subsequent suppressor cell generation. Cell Immunol 83:83–91
Steinberg T, Poucher RL, Sarin RK, Rees RB, Gwinup G (1970) Oral administration of d-ribose in diabetes mellitus. Diabetes 19:11–16
Ulrich F (1983) Inhibition by specific monosaccharides of interleukin 2-induced thymocyte proliferation. Cell Immunol 80:241–256
Wagner DR, Zöllner N (1991) McArdle's disease: successful symptomatic therapy by high dose oral administration of ribose. Klin Wochenschr 69:92
Zimmer H-G, Gerlach E (1978) Stimulation of myocardial adenine nucleotide biosynthesis by pentoses and pentitols. Plugers Arch 376:223–227
Zöllner N, Reiter S, Gross M, Pongratz D, Reimers CD, Gerbitz K, Paetzke I, Deufel T, Hübner G (1986) Myoadenylate deaminase deficiency: successful symptomatic therapy by high dose oral administration of ribose. Klin Wochenschr 64:1281–1290
Zunica G, Marini M, Brunelli MA, Chiricolo M, Franceschi C (1986) d-ribose inhibits DNA repair synthesis in human lymphocytes. Biochem Biophys Res Comm 138:673–678
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Pliml, W., von Arnim, T. & Hammer, C. Effects of therapeutic ribose levels on human lymphocyte proliferation in vitro. Clin Investig 71, 770–773 (1993). https://doi.org/10.1007/BF00190316
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00190316