Abstract
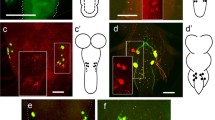
We describe the development of 20 sensory organs in the embryonic Drosophila head, which give rise to 7 sensory nerves of the peripheral nervous system (PNS), and 4 ganglia of the stomatogastric nervous system (SNS). Using these neural elements and the optic lobes as well as expression domains of the segment polarity gene engrailed in the wild-type head of Drosophila embryos as markers we examined the phenotype of different mutants which lack various and distinct portions of the embryonic head. In the mutants, distinct neural elements and engrailed expression domains, serving as segmental markers, are deleted. These mutants also affect the optic lobes to various degrees. Our results suggest that the optic lobes are of segmental origin and that they derive from the ocular segment anteriorly adjacent to the antennal segment of the developing head.
Similar content being viewed by others
References
Baker NE (1988) Localization of transcripts from the wingless gene in whole Drosophila embryos. Development 103:289–298
Bier E, Vässin H, Shepherd S, Lee K, McCall K, Barbel S, Ackerman L, Carretto R, Uemura T, Grell E, Jan LY, Jan YN (1989) Searching for pattern and mutation in the Drosophila genome with a P-lacZ vector. Genes Dev 3:1273–1287
Campos-Ortega JA, Hartenstein V (1985) The embryonic development of Drosophila melanogaster. Springer, Berlin Heidelberg New York
Casanova J, Struhl G (1993) The torso receptor localizes as well as transduces the spatial signal specifying terminal body pattern in Drosophila. Nature 362:152–155
Cheyette BN, Green PJ, Martin K, Garren H, Hartenstein V, Zipursky SL (1994) The Drosophila sine oculis locus encodes a homeodomain-containing protein required for the development of the entire visual system. Neuron 12:977–996
Cohen SM, Jürgens G (1990) Mediation of Drosophila head development by gap-like segmentation genes. Nature 346:482–485
Dalton D, Chadwick R, McGinnis W (1989) Expression and embryonic function of empty spiracles: a Drosophila homeo box gene with two patterning functions on the anterior-posterior axis of the embryo. Genes Dev 3:1940–1956
Dorresteijn AWC, O'Grady B, Fischer A, Porchet-Henneré E, Boilly-Marer Y (1993) Molecular specification of cell lines in the embryo of Platynereis (Annelida). Roux's Arch Dev Biol 202:260–269
Finkelstein R, Perrimon N (1990) The orthodenticle gene is regulated by bicoid and torso and specifies Drosophila head development. Nature 346:485–488
Fischer A (1985) Reproduction and postembryonic development of the annelid, Platynereis dumerilii. Film C1577., Institut für den wissenschaftlichen Film, Göttingen, Germany
Fujita SC, Zipursky SL, Benzer S, Ferrus A, Shotwell SL (1982) Monoclonal antibodies against Drosophila nervous system. Proc Natl Acad Sci USA 79:7929–7933
González-Gaitán M, Rothe M, Wimmer EA, Taubert H, Jäckle H (1994) Redundant functions of the genes knirps and knirps-related for the establishment of anterior Drosophila head structures. Proc Natl Acad Sci USA 91:8567–8571
Green P, Hartenstein AY, Hartenstein V (1993) The embryonic development of the Drosophila visual system. Cell Tissue Res 273:583–598
Haget A (1977) L'embryologie des insectes. In: Grassé P-P (ed) Traité de Zoologie, vol VIII Fascicule V-B. Masson, Paris, pp 134–262
Hartenstein V, Tepass U, Gruszynski E (1994) Embryonic development of the stomatogastric nervous system in Drosophila. J Comp Neurol 350:367–381
Horridge GA (1965) The Arthropoda. In: Bullock TH, Horridge GA (eds) Structure and function in the nervous systems of inverterbrates, vol 2. Freeman WH and Company, San Francisco, pp 801–1270
Jürgens G, Hartenstein V (1993) The terminal regions of the body pattern. In: Bate M, Martinez-Arias A (eds) The development of Drosophila melanogaster, vol 1. CSHL Press, Cold Spring Harbor pp 687–746
Jürgens G, Lehmann R, Schardin M, Nüsslein-Volhard C (1986) Segmental organization of the head in the embryo of Drosophila melanogaster. Roux's Arch Dev Biol 195:359–377
Lee JJ, Kessler DP von, Parks S, Beachy PA (1992) Secretion and localized transcription suggest a role in positional signalling for products of the segmentation gene hedgehog. Cell 71:33–50
Martin JR, Raibaud A, Ollo R (1994) Terminal pattern elements in Drosophila embryo induced by the torso-like protein Nature 367:741–745
Ouelette RJ, Valet JP, Coté S (1992) Expression of gooseberry-proximal in the Drosophila developing nervous system responds to cues provided by segment polarity genes. Roux's Arch Dev Biol 201:157–168
Patel NH, Martin-Blanco E, Coleman KG, Poole SJ, Ellis MC, Kornberg TB. Goodman CS (1989) Expression of engrailed proteins in arthropods, annelids, and chordates. Cell 58:955–968
Penzlin H (1985) Stomatogastric nervous system. In: Kerkut GA, Gilbert LI (eds) Comprehensive insect physiology biochemistry and pharmacology, vol 5. Pergamon Press, Oxford, pp 371–406
Pignoni F, Baldarelli RM, Steingrímsson E, Diaz RJ, Patapoutian A, Merriam JR, Lengyel JA (1990) The Drosophila gene tailless is expressed at the embryonic termini and is a member of the steroid receptor superfamily. Cell 62:151–163
Pignoni F, Steingrímsson E, Lengyel JA(1992) bicoid and the terminal system activate tailless expression in the early Drosophila embryo. Development 115:239–251
Rempel JG (1975) The evolution of the insect head: an endless dispute. Questiones Entomologicae 11:7–25
Schmidt-Ott U, Technau GM (1992) Expression of en and wg in the embryonic head and brain of Drosophila indicates a refolded band of seven segment remnants. Development 116:111–125
Schmidt-Ott U, Technau GM (1994) Fate-mapping in the procephalic region of the embryonic Drosophila head. Roux's Arch Dev Biol 203:367–373
Schmidt-Ott U, Sander K, Technau GM (1994a) Expression of engrailed in embryos of a beetle and five dipteran species with special reference to the terminal regions. Roux's Arch Dev Biol 203:298–303
Schmidt-Ott U, Gonzalez Gaitan M, Jäckle H, Technau GM (1994b) Number, identity and sequence of the Drosophila head segments as revealed by neural elements and their deletion patterns in mutants. Proc Natl Acad Sci USA 91:8363–8367
Schmucker D, Taubert H, Jäckle H (1992) Formation of the Drosophila larval photoreceptor organ and its neuronal differentiation require continuous Krüppel gene activity. Neuron 9:1025–1039
Scholtz G (1994) Head segmentation in Crustacea — an immunocytochemical study. Zoology, in press
Siewing R (1963) Zum Problem der Arthropodenkopfsegmentierung. Zool Anz 170:429–468
Sprenger F, Stevens LM, Nüsslein-Volhard C (1989) The Drosophila gene torso encodes a putative receptor tyrosine kinase. Nature 338:478–483
Strecker TR, Merriam JR, Lengyel JA (1988) Graded requirement for the zygotic terminal gene, tailless, in the brain and tail region of the Drosophila embryo. Development 102:721–734
Tabata T, Eaton S, Kornberg TB (1992) The Drosophila hedgehog gene is expressed specifically in posterior compartment cells and is a target of engrailed regulation. Genes Dev 6:2635–2645
Walldorf U, Gehring WJ (1992) Empty spiracles, a gap gene containing a homeobox involved in Drosophila head development. EMBO J 11:2247–2259
Wieschaus E, Nüsslein-Volhard C, Jürgens G (1984) Mutations affecting the pattern of the larval cuticle in Drosophila melanogaster. Roux's Arch Dev Biol 193:296–307
Wimmer EA, Jäckle H, Pfeifle C, Cohen SM (1993) A Drosophila homologue of human Sp1 is a head-specific segmentation gene. Nature 366:690–694
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Schmidt-Ott, U., González-Gaitán, M. & Technau, G.M. Analysis of neural elements in head-mutant Drosophila embryos suggests segmental origin of the optic lobes. Roux's Arch Dev Biol 205, 31–44 (1995). https://doi.org/10.1007/BF00188841
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00188841