Abstract
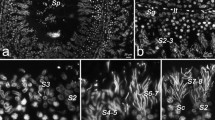
New antibody markers have allowed more refined examinations of embryogenesis. Features are being found that were overlooked in whole and sectioned embryos stained with traditional histochemical labels. Two monoclonal antibodies that recognize two different cell surface proteins in Manduca sexta label cells of the developing reproductive system. These specific immunolabels reveal that during a brief period of Manduca embryogenesis, rudiments of both male and female genital ducts are present in a single embryo. This transient phase of genital differentiation parallels the transient indifferent stage known to occur during development of reproductive systems in many vertebrate embryos. At the end of this indifferent stage, one of the two pairs of genital ducts retracts and degenerates. The dynamic expression of the two surface proteins on cells involved in morphogenesis of both the female and male reproductive systems also suggests that these proteins are important in orchestrating the specific cellular interactions that occur between mesodermal cells of the genital ducts and the nearby ventral ectoderm.
Similar content being viewed by others
References
Anderson DT (1972a) The development of holometabolous insects. In: Counce SJ, Waddington CH (eds) Developmental systems: insects. Academic Press, London New York, pp 165–242
Anderson DT (1972b) The development of hemimetabolous insects. In: Counce SJ, Waddington CH (eds) Developmental systems: insects. Academic Press, London New York, pp 95–163
Bate M (1993) The mesoderm and its derivatives. In: Bate, M. Martinez Arias A (eds) The development of Drosophila melanogaster. Cold Spring Harbor Laboratory Press, pp 1013–1090
Bieber AJ, Snow PM, Hortsch M, Patel NH, Jacobs JR, Traquina ZR, Schilling J, Goodman CS (1989) Drosophila neuroglian: A member of the immunoglobulin superfamily with extensive homology to the vertebrate neural adhesion molecule L1. Cell 59:447–460
Broadie KS, Bate M, Tublitz NJ (1991) Quantitative staging of embryonic development of the tobacco hawkmoth, Manduca sexta. Roux's Arch Dev Biol 199:327–334
Brookman JJ, Toosy AT, Shashidhara LS, White RAH (1992) The 412 retrotransposon and the development of gonadal mesoderm in Drosophila. Development 116:1185–1182
Carr JN, Taghert PH (1988) Formation of the transverse nerve in moth embryos. I. A scaffold of nonneuronal cells prefigures the nerve. Dev Biol 130:487–499
Cohen SM (1993) Imaginal disc development. In: Bate M, Martinez Arias A (eds) The development of Drosophila melanogaster. Cold Spring Harbor Laboratory Press, pp 747–841
Copenhaver PF, Taghert PH (1989) Development of the enteric nervous system is the moth. I. Diversity of cell types and the embryonic expression of FRMF amide related neuropeptides. Dev Biol 131:70–84
Demerec M (1965) Biology of Drosophila. Hafner, New York and London
Epper F, Nöthiger R (1982) Genetic and developmental evidence for a repressed genital primordium in Drosophila melanogaster. Dev Biol 94:163–175
Gilbert SF (1994) Developmental biology, Sinauer, Sunderland, MA, USA
Grenningloh G, Rehm EJ, Goodman CS (1991) Genetic analysis of growth cone guidance in Drosophila: fasciclin II functions as a neuronal recognition molecule. Cell 67:45–57
Gruenwald P (1952) Development of the excretory system. Ann NY Acad Sci 55:142–146
Harrelson AL, Goodman CS (1988) Growth cone guidance in insects: fasciclin II is a member of the immunoglobulin superfamily. Science 242:700–708
Horsfall WR, Anderson JF (1961) Suppression of male characteristics of mosquitoes by thermal means. Science 133:1830
Horsfall WR, Ronquillo MC, Patterson WH (1972) Genesis of the reproductive system of mosquitoes IV: thermal modification of Aedes stimulans. Israel J Entomol 7:73–84
Lawrence PA, Johnston P (1986) Observations on cell lineage of internal organs of Drosophila. J Embryol Exp Morphol 91:251–266
Lewis EB (1978) A gene complex controlling segmentation in Drosophila. Nature 276:565–570
Matsuda R (1976) Morphology and evolution of the insect abdomen. Pergamon Press, Oxford
Nardi JB (1990) Expression of a surface epitope on cells that link branches in the tracheal network of Manduca sexta. Development 110:682–688
Nardi JB (1992) Dynamic expression of a cell surface protein during rearrangement of epithelial cells in the Manduca wing monolayer. Dev Biol 152:161–171
Nardi JB (1993) Modulated expression of a surface epitope on migrating germ cells of Manduca sexta embryos. Development 118:967–975
Poole TJ, Steinberg MS (1982) Evidence for the guidance of pronephric duct migration by a craniocaudally traveling adhesive gradient. Dev Biol 92:144–158
Snodgrass RE (1935) Principles of insect morphology. McGraw-Hill, New York London
Sommer RJ, Steinberg PW (1994) Changes of induction and competence during the evolution of vulva development in nematodes. Science 265:114–118
Wheeler WM (1893) A contribution to insect embryology. J Morphol 8:1–160
Wieschaus E, Nöthiger R (1982) The role of the transformer genes in the development of genitalia and analia of Drosophila melanogaster. Dev Biol 90:320–334
Zackson SL, Steinberg MS (1987) Chemotaxis or adhesion gradient? Pronephric duct elongation does not depend on distant sources of guidance information. Dev Biol 124:418–422
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Nardi, J.B., Cattani, E.G. Expression of a cell surface protein during morphogenesis of the reproductive system in Manduca sexta embryos. Roux's Arch Dev Biol 205, 21–30 (1995). https://doi.org/10.1007/BF00188840
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00188840