Abstract
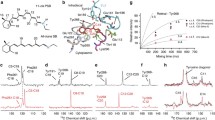
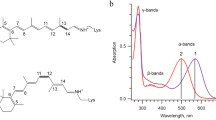
The photoreaction of rhodopsin regenerated with 11-cis-13-demethyl-retinal was investigated by FTIR difference spectroscopy. The measurements show that the chromophore experiences different twists in the modified bathorhodopsin as compared to normal bathorhodopsin and that the twists are relaxed in the additional intermediate batho-lumirhodopsin. Whereas the missing methyl group influences the lumimetarhodopsin-I transition, a metarhodopsin-I-metarhodopsin-II difference spectrum very similar to that of unmodified rhodopsin is observed. The significance of the steric interaction for regulating the photoreaction is discussed.
Similar content being viewed by others
Abbreviations
- 9-H-:
-
9-demethyl
- 13-H:
-
13-demethyl
- 5,6-H2 :
-
5,6-dihydro
- HOOP:
-
hydrogen-out-of-plane
- FTIR:
-
Fourier transform infrared
References
Albeck A, Friedman N, Ottolenghi M, Sheves M, Einterz CM, Hug SJ, Lewis JW Kliger DS (1989) Photolysis intermediates of the artificial visual pigment cis-5,6-dihydro-isorhodopsin. Biophys J 55:233–241
Bagley KA, Balogh-Nair V, Croteau AA, Dollinger G, Ebrey TG, Eisenstein L, Hong MK, Nakanishi K, Vittitow J (1985) Fourier-Transform infrared difference spectroscopy of rhodopsin and its photoproducts at low temperature. Biochemistry 24:6055–6071
Derguini F, Nakanishi K (1986) Synthetic rhodopsin analogs. Photobiochem Photobiophys 13:259–283
Ebrey T, Tsuda M, Sasenrath G, West JL, Waddell WH (1980) Light activation of bovine rod phosphodiesterase by non-physiological visual pigments. FEBS Lett 116:217–219
Emeis D, Kühn H, Reichert J, Hofmann DP (1982) Complex formation between metarhodopsin II and GTP-binding protein in bovine photoreceptor membranes leads to a shift of the photoproduct equilibrium. FEBS Lett 143:29–34
Eyring G, Curry B, Mathies RA, Fransen R, Palings I, Lugtenburg J (1980) Interpretation of the resonance Raman spectrum of bathorhodopsin based on visual pigment analogues. Biochemistry 19:2410–2418
Fahmy K Großjean MF, Siebert F, Tavan P (1989) The photoisomerization in bacteriorhodopsin studied by FTIR linear dichroism and photoselection experiments combined with quantum-chemical theoretical analysis. J Mol Struct 214:257–288
Fukada Y, Shichida Y, Yoshizawa T Ito M, Kodama A, Tsukida K (1984) Studies on structure and function of rhodopsin by use of cyclopentatrienylidene 11-cis-locked-rhodopsin. Biochemistry 23:5826–5832
Gärtner W, Hopf H, Hull WE, Oesterhelt D, Scheutzow D, Towner P (1980) On the photoisomerization of 13-demethyl retinal. Tetrahedron Lett 21:347–350
Ganter UM (1989) Dissertation. Albert-Ludwig-Universität, Freiburg
Ganter UM, Gärtner W, Siebert F(1988) Rhodopsin-lumirhodopsin phototransition of bovine rhodopsin investigated by FTIR difference spectroscopy. Biochemistry 27:7480–7488
Ganter UM, Schmid ED, Perez-Sala D,Rando RR, Siebert F (1989) Removal of the 9-methyl group of retinal inhibits signal transduction in the visual process. A Fourier transform infrared and biochemical investigation. Biochemistry 28:5954–5962
Longstaff C, Calhoon RD, Rando RR (1986) Deprotonation of the Schiff base of rhodopsin is obligate in the activation of the G protein. Proc Natl Acad Sci USA 83:4209–4213
Mathies RA, Smith SO, Palings I (1987) Determination of retinal chromophore structure in rhodopsins. In: Spiro TG (ed) Biological application of Raman spectrometry, vol 2: Resonance Raman spectra of polyenes and aromatics. Wiley, Chichester, pp 59–108
Palings I, Pardoen JA, Van den Berg E, Winkel C, Lugtenburg J, Mathies RA (1987) Assignment of fingerprint vibrations in the resonance Raman spectra of rhodopsin, isorhodopsin and bathorhodopsin: implications for the chromophore structure and environment. Biochemistry 26:2544–2556
Palings I, Van den Berg EMM, Lugtenburg J, Mathies RA (1989) Complete assignment of the hydrogen out-of-plane wagging vibrations of bathorhodopsin: chromophore structure and energy storage in the primary photoproduct of vision. Biochemistry 28:1498–1507
Shichida Y, Kropf A, Yoshizawa T (1981) Photochemical reactions of 3-demethyl visual pigment analogues at low temperature. Biochemistry 20:1962–1968
Siebert F, Mäntele W Gerwert K (1983) Eur J Biochem 130:119–127
Stryer L (1986) Cyclic GMP cascade of vision. Ann Rev Neurosci 9:87–119
Author information
Authors and Affiliations
Additional information
Offprint requests to: F. Siebert
Rights and permissions
About this article
Cite this article
Ganter, U.M., Gärtner, W. & Siebert, F. The influence of the 13-methyl group of the retinal on the photoreaction of rhodopsin revealed by FTIR difference spectroscopy. Eur Biophys J 18, 295–299 (1990). https://doi.org/10.1007/BF00188042
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00188042