Summary
In Xenopus, we investigated the origin of enteric neurones and their distribution in relation to the extracellular matrix (ECM) components, fibronectin (FN) and tenascin (TN). Enteric neurone precursor cells originate from the anterior trunk neural crest (NC). They migrate along the ventromedial NC pathway (between somites and neural tube/notochord) into the primitive gut (via the dorsal mesentery/lateral plate mesoderm) where they differentiate into enteric neurones. NC cells were identified during their migration and in the gut using the X. laevis — X. borealis nuclear marker system. The neuronal character of NC cells in the gut could be demonstrated immunohistochemically with a monoclonal antibody against the HNK-1 epitope. This antibody is superior to N-CAM and neurofilament antibodies which proved insufficient in Xenopus.
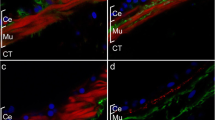
In early tadpoles (stage 45), enteric neurones occurred frequently in the mesenchymal lining of the oesophagus, either singly or in groups of two to three cells. In more distal portions of the digestive tract, enteric neurones were rarely found. In metamorphosing tadpoles (stage 62/63), enteric neurones were scattered singly beneath the mucosa, or formed small aggregates between the inner and outer muscle layer throughout the length of the digestive tract. The neurones occurred in positions corresponding to the myenteric and submucosal plexus of higher vertebrates.
The distribution of enteric neurones was studied in relation to fibronectin (FN) and tenascin (TN), glycoproteins of the ECM, which support (FN) and inhibit (TN) amphibian NC cell migration. Using immunohisto-chemistry, FN was found during NC cell migration in ECM spaces along the ventromedial pathway, and in the gut between the mucosa and the muscle layers, where it would be able to support adhesion and migration of NC cells. TN, in contrast, appeared much later than FN, both in the dorsal trunk and also ventrally, in the gut. In older tadpoles, TN was present in the mesenchyme and muscle layers of the digestive tract, where it might have an inhibiting influence on the migration of enteric neurones within the gut wall.
Similar content being viewed by others
References
Andrew A (1971) The origin of intramural ganglia. IV. The origin of enteric ganglia: a critical review and discussion of the present state of the problem. J Anat 108:169–184
Allan IJ, Newgreen DF (1980) The origin and differentiation of enteric neurons of the intestine of the fowl embryo. Am J Anat 157:137–154
Aufderheide E, Ekblom P (1988) Tenascin during gut development: Appearance in the mesenchyme, shift in molecular forms, and dependence on epithelial-mesenchymal interactions. J Cell Biol 107:2341–2349
Babák E (1906) Experimentelle Untersuchungen über die VariabiLität der Verdauungsröhre. Arch Entwicklungsmech Org 21:611–702
Boucaut J-C, Darribère T, Poole TJ, Aoyama H, Yamada KM, Thiery J-P (1984) Biologically active synthetic peptides as probes of embryonic development: A competitive peptide inhibitor of fibronectin function inhibits gastrulation in amphibian embryos and neural crest cell migration in avian embryos. J Cell Biol 99:1822–1830
Bronner-Fraser M (1986) Analysis of the early stages of trunk neural crest migration using monoclonal antibody HNK-1. Dev Biol 115:44–55
Bronner-Fraser M (1988) Distribution and function of tenascin during cranial neural crest development in the chick. J Neurosci Res 21:135–147
Burnstock G (1986) Autonomic neuromuscular junctions: current developments and future directions. J Anat 146:1–30
Chiquet-Ehrismann R, Mackie EJ, Pearson CA, Sakakura T (1986) Tenascin: an extracellular matrix protein involved in tissue interactions during fetal development and oncogenesis. Cell 47:131–139
Chiquet-Ehrismann R, Kalla P, Pearson CA, Beck K, Chiquet M (1988) Tenascin interferes with fibronectin action. Cell 53:383–390
Ciment G, Weston JA (1983) Enteric neurogenesis by neural crest-derived branchial and mesenchymal cells. Nature 305:424–427
Detwiler SR (1937) Observations upon the migration of neural crest cells, and upon the development of the spinal ganglia and vertebral arches in Amblystoma. Am J Anat 61:63–94
Epperlein HH, Halfter W, Tucker RP (1988) The distribution of fibronectin and tenascin along the migratory pathways of the neural crest in the trunk of amphibian embryos. Development 103:743–756
Fox H, Mahoney R, Bailey E (1970) Aspects of the ultrastructure of the alimentary canal and associated glands of the Xenopus laevis larva. Arch Biol (Liège) 81:21–50
Fox H, Bailey E, Mahoney R (1972) Aspects of the ultrastructure of the alimentary canal and respiratory ducts in Xenopus laevis larvae. J Morphol 138:387–406
Gabella G (1976) Structure of the autonomic nervous system. Chapman and Hall, London
Gabella G (1979) Innervation of the gastrointestinal tract. Int Rev Cytol 59:129–193
Geigy R, Engelmann F (1954) Beitrag zur Entwicklung und Metamorphose des Darmes bei Xenopus laevis Daud. Rev Suisse Zool 61:335–347
Gershon MD (1981) The enteric nervous system. Annu Rev Neuroscience 4:227–272
Greenberg JH, Seppä S, Seppä H, Hewitt AT (1981) Role of collagen and fibronectin in neural crest cell adhesion and migration. Dev Biol 87:251–266
Gunn M (1951) A study of the enteric plexuses in somme amphibians. Q J Microsc Sci 92:55–78
Halfter W, Chiquet-Ehrismann R, Tucker R (1989) The effect of tenascin and embryonic basal lamina on the behavior and morphology of neural crest cells in vitro. Dev Biol 132:14–25
Hourdry J, Dauca M (1977) Cytological and cytochemical changes in the intestinal epithelium during anuran metamorphosis. Int Rev Cytol [Suppl 5] 337–385
Hynes RO (1981) Fibronectin and its relation to cellular structure and behaviour. In: Hay ED (ed) Cell biology of extracellular matrix. Plenum Press, New York, pp 295–334
Ishizuya-Oka A, Shimozawa A (1987a) Development of the connective tissues in the digestive tract of the larval and metamorphosing Xenopus laevis. Anat Anz 164:81–93
Ishizuya-Oka A, Shimozawa A (1987b) Ultrastructural changes in the intestinal connective tissue of Xenopus laevis during metamorphosis. J Morphol 193:13–22
Junqueira LC, Carneiro J (1983) Basic Histology. Lange, Los Altos
Kemp NE (1951) Development of intestinal coiling in anuran larvae. J Exp Zool 116:259–287
Kordylewski L (1983) Light and electron microscopic observations of the development of intestinal musculature in Xenopus. Z Mikrosk Anat Forsch 97:719–734
Krotoski D, Bronner-Fraser M (1986) Mapping of neural crest pathways in Xenopus laevis. In: Slavkin HC (ed) Progress in Development Biology, part B. Alan R Liss, New York, pp 229–233
Krotoski D, Domingo C, Bronner-Fraser M (1986) Distribution of a putative cell surface receptor for fibronectin and laminin in the avian embryo. J Cell Biol 103:1061–1071
Krotoski DM, Fraser SE, Bronner-Fraser M (1988) Mapping of neural crest pathways in Xenopus laevis using inter- and intraspecific cell markers. Dev Biol 127:119–132
Lander AD, Fujii DK, Reichardt LT (1985) Laminin is associated with the neurite outgrowth-promoting factor found in conditioned medium. Proc Natl Acad Sci USA 82:2183–2187
Le Douarin NM (1969) Particularités du noyau interphasique chez la Caille japonaise (Coturnix coturnix japonica). Utilisation de ces particularités comme ‘marquage biologique’ dans les recherches sur les interactions tissulaires et les migrations cellulaires au cours de l'ontogenèse. Bull Biol Fr Belg 103:435–452
Le Douarin NM (1982) The neural crest. Cambridge University Press, Cambridge
Le Douarin NM, Teillet M-A (1973) The migration of neural crest cells to the wall of the digestive tract in avian embryo. JEEM 30:31–48
Le Douarin NM, Teillet M-A (1974) Experimental analysis of the migration and differentiation of neuroblasts of the autonomic nervous system and of neurectodermal mesenchymal derivatives, using a biological cell marking technique. Dev Biol 41:162–184
Le Douarin NM, Teillet M-A, Le Lièvre C (1977) Influences of the tissue environment on the differentiation of neural crest cells. In: Lash JW, Burger MM (eds) Cell and tissue interactions. Raven Press, New York, pp 11–27
Le Douarin NM, Cochard P, Vincent M, Duband J-L, Tucker GC, Teillet M-A, Thiery J-P (1984) Nuclear, cytoplasmic, and membrane markers to follow neural crest cell migration: a comparative study. In: Trelstad RL (ed) The role of extracellular matrix in development. Liss, New York, pp 373–398
Macmillan GJ (1976) Melanoblast-tissue interactions and the development of pigment pattern in Xenopus larvae. JEEM 35:463–484
Mackie EJ, Tucker RP, Halfter W, Chiquet-Ehrismann R, Epperlein HH (1988) The distribution of tenascin coincides with pathways of neural crest cell migration. Development 102:237–250
Newgreen DF, Erickson CA (1986) The migration of neural crest cells. Int Rev Cytol 103:89–145
Newgreen DF, Gibbins IL, Sauter J, Wallenfels B, Wütz R (1982) Ultrastructural and tissue-culture studies on the role of fibronectin, collagen and glycosaminoglycans in the migration of neural crest cells in the fowl embryo. Cell Tissue Res 221:521–549
Nieuwkoop PD, Faber J (1975) Normal table of Xenopus laevis (Daudin). Amsterdam, North Holland
Nordlander RH (1989) HNK-1 marks earliest axonal outgrowth in Xenopus. Dev Brain Res 50:147–153
Perris R, v Boxberg Y, Löfberg J (1988) Local embryonic matrices determine region-specific phenotypes in neural crest cells. Science 241:86–89
Rintoul JR (1960) The comparative morphology of the enteric nerve plexuses. Thesis, University of St. Andrews
Rovasio RA, Delouvée A, Yamada KM, Timpl R, Thiery J-P (1983) Neural crest cell migration: requirements for exogenous fibronectin and high cell density. J Cell Biol 96:462–473
Sadaghiani B, Thiébaud CH (1987) Neural crest development in the Xenopus laevis embryo, studied by interspecific transplantation and scanning electron microscopy. Dev Biol 124:91–110
Steinberg MS (1957) A nonnutrient culture medium for amphibian embryonic tissue. Carnegie Inst Washington Yearb 56:347–348
Stern CD, Norris WF, Bronner-Fraser M, Carlson GJ, Faissner A, Keynes RJ, Schachner M (1989) J1/tenascin-related molecules are not responsible for the segmented pattern of neural crest cells or motor axons in the chick embryo. Development 107:309–319
Tan S-S, Crossin KL, Hoffman S, Edelman GM (1987) Asymmetric expression in somites of cytotactin and its proteoglycan ligand is correlated with neural crest cell distribution. Proc Natl Acad Sci USA 84:7977–7981
Tennyson VM, Pham TD, Rothman TP, Gershon MD (1986) Abnormalities of smooth muscle, basal laminae, and nerves in the aganglionic segments of the bowel of lethal spotted mutant mice. Anat Rec 215:267–281
Thiébaud CH (1983) A reliable new cell marker in Xenopus. Dev Biol 98:245–249
Thiery JP, Duband JL, Tucker GC (1985) Migrations in the vertebrate: role of cell adhesion and tissue environment in the patterning of the embryo. Ann Rev Cell Biol 1:91–113
Tucker GC, Aoyama H, Lipinski M, Turz T, Thiery JP (1984) Identical reactivity of monoclonal antibodies HNK-1 and NC-1: Conservation in vertebrates on cells derived from neural primordium and on some leukocytes. Cell Differ 14:223–230
Tucker GC, Ciment G, Thiery JP (1986) Pathways of avian neural crest cell migration in the developing gut. Dev Biol 116:439–450
Tucker GC, Delarue M, Zada S, Boucaut J-C, Thiery JP (1988) Expression of the HNK-1/NC-1 epitope in early vertebrate neurogenesis. Cell Tissue Res 251:457–465
Tucker RP (1986) The role of glycosaminoglycans in anuran pigment cell migration. JEEM 92:145–164
Tucker RP, Erickson CA (1986) The control of pigment cell pattern formation in the California newt, Taricha torosa. JEEM 97:141–168
Vincent M, Thiery JP (1984) A cell surface marker for neural crest and placodal cells: further evolution in peripheral and central nervous system. Dev Biol 103:468–481
Vogel DL, Model PG (1977) Development of the sympathetic system in the Mexican Axolotl, Ambystoma mexicanum. Dev Biol 56:76–96
Wittekind D, Schmidt G, Kretschmer V (1987) A simple and reproducible staining procedure to assess characteristic effects of some fixatives. Acta Histochem [Suppl. XXXIV] 143–151
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Epperlein, HH., Krotoski, D., Halfter, W. et al. Origin and distribution of enteric neurones in Xenopus . Anat Embryol 182, 53–67 (1990). https://doi.org/10.1007/BF00187527
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00187527