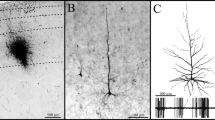
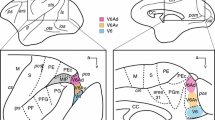
Abstract
Fluorescent somatopetal tracers were used to infiltrate, by diffusion rather than injections, the dorsolateral cortex of one hemisphere in rats. In different animals the tracers penetrated into the cortex to different depths. We found several interesting features of the commissural system: first, there were no areas without commissural neurons. At least a few labelled cell bodies were present in a single-cell layer also in “acallosal” cortical areas. Secondly, there is a considerable variety of laminar distribution patterns of labelled perikarya in different areas. Thirdly, some cortical fields, which cytoarchitecturally appear uniform, can be subdivided according to different distributions of cell bodies with commissural projections. Fourthly, when only supragranular layers were infiltrated, labelled cell bodies were present mainly in the supragranular layers of the contralateral cortex. Infiltration of the first layer alone did not label any neurons in the contralateral cortex but did label neurons in layer VIb ipsilaterally. In the subcortex, the labelled perikarya were found in the structures already known to project directly to the cortex. In rats with the tracer restricted mainly to the supragranular layers, a conspicuously reduced labelling was found in the basal forebrain and the thalamus. In the thalami of those animals, labelled neurons were found only in paralamellar nuclei. The high sensitivity of the tracer used, together with infiltration of the entire dorsolateral cortex, allows us to conclude that probably all sources of innervation of the isocortex in rats have been seen.
Similar content being viewed by others
References
Aschoff A, Holländer H (1982) Fluorescent compounds as retrograde tracers compared with horseradish peroxidase (HRP). I. A parametric study in the central visual system of the albino rat. J Neurosci Methods 6:179–197
Audinat E, Condé F, Crépel F (1988) Cortico-cortical connections of the limbic cortex of the rat. Exp Brain Res 69:439–443
Beckstead RM (1979) An autoradiographic examination of cortical and subcortical projections of the mediodorsal projection (prefrontal) cortex in the rat. J Comp Neurol 184:43–62
Cowan WM, Gottlieb DI, Hendrickson AE, Price JL, Woolsey TA (1972) The autoradiographic demonstration of axonal connections in the central nervous system. Brain Res 37:21–51
Cusick CG, Lund RD (1982) Modification of visual callosal projections in rats. J Comp Neurol 212:385–398
De Olmos J, Heimer L (1980) Double and triple labelling of neurons with fluorescent substances: the study of collateral pathways in the ascending raphe system. Neurosci Lett 19:7–12
Divac I (1975) Magnocellular nuclei of the basal forebrain project to neocortex, brain stem, and olfactory bulb. Review of some functional correlates. Brain Res 93:385–398
Divac I (1981) Cortical projections of the magnocellular nuclei of the basal forebrain: a reinvestigation. Neuroscience 6:983–984
Divac I, Mogensen J (1990) Long-term labelling of neurons. Brain Res 524:339–341
Divac I, LaVail JH, Rakic P, Winston KR (1977) Heterogeneous afferents to the inferior parietal lobule of the rhesus monkey revealed by the retrograde transport method. Brain Res 123:197–207
Divac I, Kosmal A, Björklund A, Lindvall O (1978) Subcortical projections to the prefrontal cortex in the rat as revealed by the horseradish peroxidase technique. Neuroscience 3:785–796
Divac I, Petrovic-Minic B, Mogensen J (1984) Focal cortical seizures prevent HRP and HRP-WGA labeling only in neurons bidirectionally connected to the cortex. Brain Res 3:189–193
Divac I, Marinkovic S, Mogensen J, Schwerdtfeger W, Regidor J (1987a) Vertical ascending connections in the isocortex. Anat Embryol 175:443–455
Divac I, Mogensen J, Marinkovic S, Mårtensson R (1987b) On the projections from the neostriatum to the cerebral cortex: the “displaced” neurons. Neuroscience 21:197–205
Dunker RO, Harris AB, Jenkins DP (1976) Kinetics of horseradish peroxidase migration through cerebral cortex. Brain Res 118:199–217
Herkenham M (1979) The afferent and efferent connections of the ventromedial thalamic nucleus in the rat. J Comp Neurol 183:487–518
Herkenham M (1986) New perspectives on the organization and evolution of nonspecific thalamocortical projections. In: Jones EG, Peters A (eds) Cerebral cortex, vol 5. Plenum Press, New York, pp 403–445
Hübener M, Boltz J (1988) Morphology of identified neurons in layer 5 of rat visual cortex. Neurosci Lett 94:76–81
Innocenti GM (1986) General organization of callosal connections in the cerebral cortex. In: Jones EG, Peters A (eds) Cerebral cortex, vol V. Plenum Press, New York, pp 291–353
Ivy GO, Gould HJ III, Killackey HP (1984) Variability in the distribution of callosal projection neurons in the adult rat parietal cortex. Brain Res 306:53–61
Jacobson S, Trojanowski JQ (1974) The cells of origin of the corpus callosum in rat, cat and rhesus monkey. Brain Res 74:149–155
Jones EG (1975) Possible determinants of the degree of retrograde neuronal labelling with horseradish peroxidase. Brain Res 85:249–253
Jouandet ML, Garey LJ, Lipp H-P (1984) Distribution of the cells of origin of the corpus callosum and anterior commissure in the marmoset monkey. Anat Embryol 169:45–59
Jouandet ML, Lachat J-J, Garey LJ (1985) Distribution of the neurons of origin of the great cerebral commissures in the cat. Anat Embryol 171:105–120
Jouandet ML, Lachet J-J, Garey LJ (1986) Topographic distribution of callosal neurons and terminals in the cerebral cortex of the cat. Anat Embryol 173:323–342
Kristensson K (1970) Transport of fluorescent protein tracer in peripheral nerves. Acta Neuropathol (Berl) 16:293–300
Kuypers HGJM, Huisman AM (1984) Fluorescent neuronal tracers. In: Advances in cellular neurobiology, vol 5. Academic Press, New York, pp 307–340
LaVail JH, LaVail MM (1972) Retrograde axonal transport in the central nervous system. Science 176:1416–1417
Leichnetz GR (1982) Connections between the frontal eye field and pretectum in the monkey. An anterograde-retrograde study using HRP gel and TMB neurohistochemistry. J Comp Neurol 207:392–402
Lipp HP, Schwegler H (1980) Improved transport of horseradish peroxidase after injection with a non-ionic detergent (Nonidet p-40) into mouse cortex and observations on the relationship between spread at the injection site and amount of transported label. Neurosci Lett 20:49–54
Luiten PGM, Gaykama RPA, Traber J, Spencer DG Jr (1987) Cortical projection patterns of magnocellular basal nucleus subdivisions as revealed by anterogradely transported Phaseolus vulgaris leucoagglutinin. Brain Res 413:229–250
Markowitsch HJ, Guldin WO (1983) Heterotopic interhemispheric cortical connections in the rat. Brain Res Bull 10:805–810
Mårtensson R, Björklund A (1984) Low-power photography in the fluorescence microscope using an automatic dark-field condenser-scanner. In: Björklund A, Hökfelt T (eds) Handbook of chemical neuroanatomy, vol 2. Classical transmitters in the CNS. Part 1. Elsevier, Amsterdam, pp 380–386
McBride RL, Feringa ER, Garver MK, Williams JK Jr (1990) Retrograde transport of Fluoro-Gold in corticospinal and rubrospinal neurons 10 and 20 weeks after T-9 spinal cord transsection. Exp Neurol 108:83–85
Mercier BE, Legg CR, Glickstein M (1990) Basal ganglia and cerebellum receive different somatosensory information in rats. Proc Natl Acad Sci USA 87:4388–4392
Miller MW, Vogt BA (1984) Heterotopic and homotopic callosal connections in rat visual cortex. Brain Res 297:75–89
Olavarria J, Van Sluyters RC (1983) Widespread callosal connections in infragranular visual cortex of the rat. Brain Res 279:233–237
Paxinos G, Watson C (1986) The rat brain in stereotaxic coordinates, 2nd edn. Academic Press, Sydney
Peyronnard J-M, Charron L (1983) Decreased horseradish peroxidase labelling in deafferented spinal motoneurons of the rat. Brain Res 275:203–214
Preuss TM, Goldman-Rakic P (1987) Crossed corticothalamic and thalamocortical connections of macaque prefrontal cortex. J Comp Neurol 257:269–281
Ribak CE (1977) A note on the laminar organization of rat visual cortical projections. Exp Brain Res 27:413–418
Rieck RW, Carey RG (1985) Organization of the rostral thalamus in the rat: Evidence for connections to layer I of visual cortex. J Comp Neurol 234:137–154
Rüttgers K, Aschoff A, Frianf E (1990) Commissural connections between the auditory cortices of the rat. Brain Res 509:71–79
Sarter M, Markowitsch HJ (1985) Convergence of intra- and interhemispheric cortical afferents: lack of collateralization and evidence for a subrhinal cell group projecting heterotopically. J Comp Neurol 236:283–296
Schmued LC (1990) Fluoro-Gold and 4-acetamido-4′-isothiocyanostilbene-2, 2′-disulfonic acid: use of substituted stilbenes in neuroanatomical studies. In: Conn PM (ed) Quantitative microscopy. Methods in neuroscience, vol 3. Academic Press, New York, pp 317–330
Schmued LC, Fallon JH (1986) Fluoro-Gold: a new fluorescent retrograde axonal tracer with numerous unique properties. Brain Res 337:147–154
Singer W, Holländer H, Vanegas H (1977) Decreased peroxidase labelling of lateral geniculate neurons following deafferentation. Brain Res 120:133–137
Vanegas H, Holländer H, Distel H (1978) Early stages of uptake and transport of horseradish peroxidase by cortical structures, and its use for the study of local neurons and their processes. J Comp Neurol 177:193–212
Wiley RG, Blessing WW, Reis DJ (1982) Suicide transport: destruction of neurons by retrograde transport of ricin, abrin and modeccin. Science 216:889–890
Wise SP, Jones EG (1976) The organization and postnatal development of the commissural porjection of the rat somatosensory cortex. J Comp Neurol 168:313–343
Zaborsky L, Wolff JR (1982) Distribution patterns and individual variations of callosal connections in the albino rat. Anat Embryol 165:213–232
Zilles K (1985) The cortex of the rat. A stereotaxic atlas. Springer, Berlin Heidelberg New York
Zilles K (1990) Anatomy of the neocortex: cytoarchitecture and myeloarchitecture. In: Kolb B, Tees RC (eds) The cerebral cortex of the rat. MIT Press, Cambridge, USA, pp 77–112
Zilles K, Zilles B, Schleicher A (1980) A quantitative approach to cytoarchitectonics. VI. The areal pattern of the cortex of the albino rat. Anat Embryol 159:335–360
Zilles K, Wree A, Schleicher A, Divac I (1984) The monocular and binocular subfields of the rat's primary visual cortex. A quantitative morphological approach. J Comp Neurol 226:391–402
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Divac, I., Regidor, J., Milosevic, S. et al. Afferents to different layers of the dorsolateral isocortex in rats. Anat Embryol 192, 63–75 (1995). https://doi.org/10.1007/BF00186992
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00186992