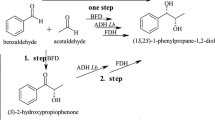
Abstract
Dehydrogenation of 2-trimethylsilyl-1-propanol (1) was carried out with horse liver alcohol dehydrogenase (HLADH, EC 1.1.1.1). It was found that the hydrogenation of 1 proceeded enantioselectively with only HLADH and a catalytic amount of NAD+ due to in-situ NAD+ regeneration based on a specific property of β-carbonylsilanes. That is, (+)-1 was enantioselectively dehydrogenated by HLADH to 2-trimethylsilyl-1-propanal, which was spontaneously degraded by addition of water into trimethylsilanol and n-propanal. Then, NAD+ was regenerated through HLADH-catalyzed reduction of n-propanal to n-propanol. On the other hand, dehydrogenation of the carbon analogue of 1 was negligible with a catalytic amount of NAD+, indicating that the in-situ NAD+ regeneration was not available without the specific property of organosilicon compounds. Other primary β-hydroxysilanes having different substituents on the chiral center or on the silicon atom were also found to serve as substrates in enantioselective dehydrogenation by HLADH with this novel NAD+ regeneration system. Chiral recognition of HLADH toward primary alcohols is also discussed.
Similar content being viewed by others
References
Ahuja S (1979) Chemical derivatization for the liquid chromatography of compounds of pharamaceutical interest. J Chromatogr Sci 17:168–172
Boyer PD (1975) The enzymes, vol 11, 3rd edn. Academic Press, New York, pp 103–190
Chen C-S, Fujimoto Y, Girdaukas G, Sih CJ (1982) Quantitative analyses of biochemical kinetic resolutions of enantiomers. J Am Chem Soc 104:7294–7299
Davis DD, Jacocks HM (1981) Deoxymetalation reactions. The mechanisms of deoxysilylation of mono-trimethylsilyl- and bis-trimethylsilyl-substituted alcohols and a comparison to the mechanism of deoxystannylation and deoxyplumbylation. J Organomet Chem 206:33–47
Dodds DR, Jones JB (1988) Enzymes in organic synthesis. 38. Preparation of enantiomerically pure chiral hydroxydecalones via stereospecific horse liver alcohol dehydrogenase catalyzed reductions of decalindiones. J Am Chem Soc 110:577–583
Fassouane A, Laval J-M, Moiroux J, Bourdillon C (1990) Electrochemical regeneration of NAD in a plug-flow reactor. Biotechnol Bioeng 35:935–939
Fessenden RJ, Fessenden JS (1980) Trends in organosilicon biological research. Adv Organomet Chem 18:275–299
Fukui T, Zong M-H, Kawamoto T, Tanaka A (1992) Kinetic resolution of organosilicon compounds by stereoselective dehydrogenation with horse liver alcohol dehydrogenase. Appl Microbiol Biotechnol 38:209–213
Hauser CR, Hance CR (1952) Preparation and reactions of α-halo derivatives of certain tetra-substituted hydrocarbon silanes. Grignard syntheses of some silyl compounds. J Am Chem Soc 74:5091–5096
Hirschbein BL, Whitesides GM (1982) Laboratory-scale enzymatic/chemical syntheses of d- and d-β-chloratic acid and d-and l-potassium glycidate. J Am Chem Soc 104:4458–4460
Hudrlik PF, Peterson D, Rona RJ (1975) Reactions of α, β-epoxysilanes with organocuprate reagents. A new stereospecific olefin synthesis. J Org Chem 40:2263–2264
Jones JB, Taylor KE (1976) Nicotinamide coenzyme regeneration. Flavin mononucleotide (riboflavin phosphate) as an efficient, economical, and enzyme-compatible recycling agent. Can J Chem 54:2969–2973
Kawamoto T, Aoki A, Sonomoto K, Tanaka A (1989) Novel photocatalytic NAD+ recycling system with a semiconductor in organic solvent. J Ferment Bioeng 67:361–362
Lee LG, Whitesides GM (1985) Enzyme-catalyzed organic synthesis: a comparison of strategies for in situ regeneration of NAD from NADH. J Am Chem Soc 107:6999–7008
Schmidt H-L, Grenner G (1976) Coenzyme properties of NAD+ bound to different matrices through the amino group in the 6-position. Eur J Biochem 67:295–302
Soderquist JA, Brown HC (1980) Hydroboration. 56. Convenient and regiospecific route to functionalized organosilanes through the hydroboration of alkenylsilanes. J Org Chem 45:3571–3578
Tacke R, Linoh H (1989) Bioorganosilicon chemistry. In: Patai S, Rappoport Z (eds) The chemistry of organic silicon compounds. Wiley, New York, pp 1143–1206
Taylor KE, Jones JB (1976) Nicotinamide coenzyme regeneration by dihydropyridine and pyridium compounds. J Am Chem Soc 98:5689–5694
Whitesides GM, Wong C-H (1985) Enzymes as catalysts in synthetic organic chemistry. Angew Chem Int Ed Engl 24:617–638
Wichmann R, Wandrey C, Bückmann AF, Kula M-R (1981) Continuous enzymatic transformation in an enzyme membrane reactor with simultaneous NAD(H) regeneration. Biotechnol Bioeng 23:2789–2802
Zong M-H, Fukui T, Kawamoto T, Tanaka A (1991) Bioconversion of organosilicon compounds by horse liver alcohol dehydrogenase: the role of the silicon atom in enzymatic reactions. Appl Microbiol Biotechnol 36:40–43
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Tsuji, Y., Fukui, T., Kawamoto, T. et al. Enantioselective dehydrogenation of β-hydroxysilanes by horse liver alcohol dehydrogenase with a novel in-situ NAD+ regeneration system. Appl Microbiol Biotechnol 41, 219–224 (1994). https://doi.org/10.1007/BF00186963
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF00186963