Abstract
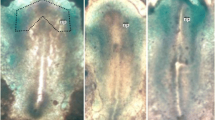
Proteoglycans are ubiquitous extracellular matrix molecules whose role in development remains poorly understood. In the developing chick limb, the nature and possible roles of a number of extracellular matrix proteins is well documented. Much less is known of the biochemical nature, and more importantly, the roles of proteoglycans. Using a panel of monoclonal antibodies (Mabs) which recognise specifie epitopes on the constituent chondroitin/dermatan sulphate chains, we show that distinct sub-populations of proteoglycans are dynamically expressed within the limb ectoderm, the ectodermal basement membrane and the limb mesenchyme. In particular, prior to chondrogensis, chondroitin-6-sulphate-rich proteoglycans containing over-sulphated domains reside predominantly within the mesenchymal extracellular matrix ECM, whilst chondroitin-4-sulphate (C-4-S) is associated with the ectodermal basement membrane and subjacent mesenchymal ECM. At stage 24, C-4-S is also localized in the prechondrogenic condensation. Concomitantly with overt chondrogenesis, the epitopes recognized by the Mabs become restricted to the chondrifying skeletal elements and the undifferentiated distal mesenchyme. The significance of these findings has yet to be elucidated.
Similar content being viewed by others
References
Archer CW, Langille RM, Teran MAF, Solursh M (1992) Myogenic potential of chick limb bud mesenchyme in micromass culture. Anat Embryol 185:299–306
Brand-Saberi B, Krenn V, Grim M, Christ B (1993) Differences in the fibronectin-dependence of migrating cell populations. Anat Embryol 187:17–26
Carney SL, Billingham MEJ, Caterson B, Ratcliffe A, Bayliss MT, Hardingham TE, Muir H (1992) Changes in proteoglycan turnover in experimental canine osteoarthritic cartilage. Matrix 12:137–147
Caterson B, Christner JE, Baker JR, Couchman JR (1985) Production and characterization of monoclonal antibodies directed against connective tissue proteoglycans. Fed Proc 44:386–393
Caterson B, Calabro T, Hampton A (1987) Monoclonal antibodies as probes for elucidating proteoglycan structure and function. In: Wight TN, Mecham RP (eds) Biology of proteoglycans. Academic Press, New York, pp 1–25
Caterson B, Mahmoodian F, Sorrell J, Hardingham T, Bayliss M, Carney S, Ratcliffe A, Muir H (1990) Modulation of native chondroitin sulphate structure in tissue development and in disease. J Cell Sci 97:411–422
Chevallier A (1978) Etude de la migration des cellules somatique dans le mesoderm somatopleural de l'ébauche de l'aile. Wilhelm Roux' Archive Entwicklungmech Org 184:57–73
Chevallier A, Kieny M, Mauger A (1976) Sur l'origine de la musculature de l'aile chez les oiseaux. CR Acad Sci (D) 282:309–311
Chevallier A, Kieny M, Mauger A (1978) Limb-somite relationship: effect of removal of the somitic mesoderm on the wing musculature. J Embryol Exp Morphol 43:263–278
Chiquet M, Eppenberger HM, Turner DC (1981) Muscle morphogenesis: evidence for an organising function of exogenous fibronectin. Dev Biol 88:220–235
Chiquet-Ehrismann R, Mackie EJ, Pearson CA, Sakakura T (1986) Tenascin: an extracellular matrix protein involved in tissue interactions during fetal development and oncogenesis. Cell 47:131–139
Christ B, Jacob HJ (1974) Über den Ursprung der Flügelmuskulatur. Experimentelle Untersuchungen an Wachtel- und Hühnerembryonen. Experientia 30:1446–1448
Couchman JR, Caterson B, Christner JE, and Baker J (1984) Mapping by monoclonal antibody detection of glycosaminoglycans in connective tissues. Nature 307:650–652
Craig F, Bentley G, Archer C (1987) The spatial and temporal pattern of collagens I and II and keratan sulphate in the developing chick metatarsophalangeal joint. Development 99:383–391
Culty M, Miyake K, Kincade PW, Silorski E, Butcher EC, Underhill C (1990) The hyaluronate receptor is a member of the CD44 (H-CAM) family of cell surface glycoproteins. J Cell Biol 111:2765–2774
Dessau WH, von der Mark K, von der Mark H (1981) Changes in the pattern of collagens and fibronectin during limb bud chondrogenesis. J Embryol Exp Morph 57:51–60
Deluca S, Heinegard D, Hascall V, Kimura J (1977). Chemical and physical changes in proteoglycans during development in chick limb bud chondrocytes grown in vitro. J Biol Chem 252:6600–6608
Goodman SL, Risse G, von der Mark K (1989) The E8 subfragment of laminin promotes locomotion of myoblasts over extracellular matrix. J Cell Biol 109:799–809
Hamburger V, Hamilton HL (1951) A series of normal stages in the development of the chick embryo. J Morphol 88:49–92
Hardingham TE, Bayliss MT (1990) Proteoglycans of articular cartilage: Changes in aging and in joint disease. Seminars in Arthritis and Rheumatism [Suppl 1]: 12–33
Hardingham TE, Fosang AJ (1992) Proteoglycans: many forms and many functions. FASEB J 6:861–870
Heinegard D, Paulsson M (1984) Structure and metabolism of proteoglycans. In: Piez KA, Reddi AK (eds) Extracellular matrix biochemistry. Plenum Press, New York, pp 277–328
Johnson GD, Davidson RS, Mcnamee KC, Russell G, Goodwin D, Halbarow EJ (1982) Fading of immunofluorescence during microscopy: a study of the phenomenon and its remedy. J Immunol Methods 55:231–242
Kimata K, Oike Y, Tani K, Shinomura T, Yamagata M, Uritani M, Suzuki S (1986) A large chondroitin sulphate proteoglycan (PG-M) synthesised before chondrogenesis in the limb of the chick embryo. J Biol Chem 261:13517–13525
Levitt D, Dorfman A (1974) Concepts and mechanisms of cartilage differentiation. In: Moscona A, Monfoy A (eds) Current topics in developmental biology 8. Academie Press, New York, pp 103–149
Mallein-Gerin F, Kosher RA, Upholt WB, Tanzer ML (1988) Temporal and spatial analysis of cartilage proteoglycan core protein gene expression during limb development by in situ hybridization. Dev Biol 126:337–345
Morris-Kay G, Tuckett F (1989) Immunohistochemical localisation of chondroitin sulphate proteoglycans and the effect of chondrotinase ABC in 9- to 11-day rat embryos. Development 106:787–798
Newgreen DF (1982) Adhesion to extracellular materials by neural crest cells at stage of initial migration. Cell Tissue Res 227:297–317
Newman SA, Patou MP, Kieny M (1981) The distal boundary of myogenic primordia in chimeric avian limb buds and its relation to an accessible population of cartilage progenitor cells. Dev Biol 84:440–448
Nishiyama A, Dahlin KJ, Stallcup WB (1991) The expression of NG2 proteoglycan in the developing rat limb. Development 111:933–944
Palmolski MJ, Goetink PF (1972) Synthesis of proteochondroitin-sulphate by normal nanomelic, and 5-bromodeoxyuridine-treated chondrocytes in cell culture. Proc Nati Acad Sci USA 69:3385–3388
Perris R, Johansson S (1987) Amphibian neural crest cell migration on purified extracellular matrix components: a chondrotin sulfate proteoglycan inhibits locomotion on fibronectin substrates. J Cell Biol 105:2511–2522
Perris R, Johansson S (1990) Inhibition of neural crest cell migration by aggregating chondroitin sulfate proteoglycans is mediated by their hyaluronan-binding region. Dev Biol 137:1–12
Perris R, Krotoski D, Lallier T, Domingo C, Sorell JM, Bronner-Fraser M (1991) Spatial and temporal changes in the distribution of proteoglycans during avian neural crest development. Development 111:583–599
Rouslahti E, Yamaguchi Y (1991) Proteoglycans as modulators of growth factor activities. Cell 64:867–869
Rutz R, Haney C, Hauschka S (1982) Spatial analysis of limb bud myogenesis: A proximo-distal gradient of muscle colony-forming cells in chick embryo leg buds. Dev Biol 90:399–411
Shinomura T, Kimata K, Oike Y, Maeda N, Yano S, Suzuki S (1984) Appearance of distinct types of proteoglycan in a well-defined temporal and spatial pattern during early cartilage formation in the chick limb. Dev Biol 103:211–220
Shinomura T, Jensen K, Yamagata M, Kimata K, Solursh M (1990) The distribution of mesenchyme proteoglycan (PG-M) during wing bud outgrowth. Anat Embryol 181:227–233
Singley CT, Solursh M (1981) The spatial distribution of hyaluronic acid and mesenchymal condensation in the embryonic chick wing. Dev Biol 84:102–120
Solursh M, Drake C, Meier S (1987) The migration of myogenic cells from the somites at the wing level in avian embryos. Dev Biol 121:389–396
Sorrell JM, Lintala A, Mahmoodian F, Caterson B (1988) Epitope-specific changes in chondroitin sulphate/dermatan sulphate proteoglycans as markers in the lymphopoietic and granulopoietic compartments of developing bursae of Fabricius. J Immunol 140:4262–4270
Sorrell JM, Mahmoodian F, Schafer IA, Davis B, Caterson B (1990) Identification of monoclonal antibodies that recognise novel epitopes in native chondroitin/dermatan sulphate glycosaminoglycan chains: their use in mapping functionally distinct domains of the human skin. J Histochem Cytochem 38:393–402
Stirpe NS, Goetinck PF (1989) Gene regulation during cartilage differentiation: a temporal and spatial expression of link protein and cartilage matrix protein in the developing limb. Development 107:23–33
Sweeney LJ, Kennedy JM, Zak R, Kokjohn K, Kelley SW (1989) Evidence for expression of a common myosin heavy chain phenotype during initial stages of avian embryogenesis. Dev Biol 133:361–374
Thompson RW, Whalen GF, Saunders KB, Hores T, D'Amore PA (1990) Heparin-mediated release of fibroblast growth factor-like activity into the circulation of rabbits. Growth Factors 3:221–229
Vasan NS, Lash JW (1979) Monomeric and aggregate proteoglycans in the chondrogenic differentiation of embryonic chick limb buds. J Embryol Exp Morphol 49:47–59
von der Mark K, Gauss V, von der Mark H, Muller P (1977) Relationships between cell shape and type of collagen synthesised as chondrocytes lose their cartilage phenotype in culture. Nature 267:531–532
Yamagata M, Kimata K, Oike Y, Tani K, Maeda N, Yoshida K, Shinomura Y, Yoneda M, Suzuki S (1987) A monoclonal antibody that specifically recognises a glucuronic acid 2-sulphate-containing determinant in intact chondroitin sulphate chains. J Biol Chem 262:4146–4152
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Fernandez-Teran, M., Bayliss, M. & Archer, C.W. Molecular heterogeneity of chondroitin sulphate in the early developing chick wing bud. Anat Embryol 188, 189–199 (1993). https://doi.org/10.1007/BF00186252
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00186252