Abstract
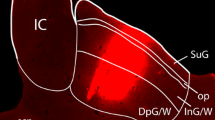
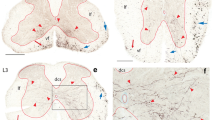
In the lateral cervical nucleus (LCN) of the cat, GABA-immunoreactive neurons and substance P-immunoreactive fibers are concentrated in the medial part of the nucleus, whereas in the monkey LCN no preferential locations have been identified. In raccoons, substance P-immunoreactive fibers display a distribution pattern similar to that in cats. However, the presence and distribution of GABA-immunoreactive neurons in the raccoon LCN has not been examined, and it is therefore not known whether raccoons are similar to cats or primates in this respect. Thus, in the present study, the raccoon LCN was examined for the presence and distribution of GABA-immunoreactive cells with respect to their numbers, locations, and sizes. The distribution of GABA-positive fibers and varicosities within the LCN was also investigated. The results of measurements of cross-sectional areas of LCN neurons indicate a trend toward decreasing cell size along the dorsolateral to medial axis of the raccoon LCN. Compared to neurons of the centrally located ventromedial division, neurons are statistically significantly larger in the dorsolateral division and smaller in the medial division of the nucleus. Cell counts in post-embedding-stained semithin sections through the nucleus revealed an average of 8,700 neurons per LCN. Approximately 4% of LCN neurons are GABA-immunoreactive. These neurons are small and most (80%) of them are located in the medial third of the LCN. In contrast, GABA-immunoreactive fibers and varicosities are present in about equal density throughout the raccoon LCN. Thus, the distributions of GABA-immunoreactive neurons and neuron sizes in the raccoon LCN conform closely to those in cats. Together with previous observations in cats and raccoons, the present findings support the notion that these small GABA-immuno-reactive neurons may be local circuit inhibitory neurons and indicate the presence of a mediolateral segregation that may be of fundamental importance for the functional organization of the carnivore LCN.
Similar content being viewed by others
References
Baker ML, Giesler GJ Jr (1984) Anatomical studies of the spinocervical tract of the rat. Somatosens Mot Res 2:1–18
Berkley KJ (1980) Spatial relationships between the terminations of somatic sensory and motor pathways in the rostral brainstem of cats and monkeys. I. Ascending somatic sensory inputs to lateral diencephalon. J Comp Neurol 193:283–317
Berkley KJ, Blomqvist A, Pelt A, Flink R (1980) Differences in the collateralization of neuronal projections from the dorsal column nuclei and the lateral cervical nucleus to the thalamus and tectum in the cat: an anatomical study using two different double-labeling techniques. Brain Res 202:273–290
Blackstad TW, Karagülle T, Ottersen OP (1990) Morforel, a computer program for two-dimensional analysis of micrographs of biological specimens, with emphasis on immunogold preparations. Comput Biol Med 20:15–34
Blessing WW (1990) Distribution of glutamate decarboxylasecontaining neurons in rabbit medulla oblongata with attention to intramedullary and spinal projections. Neuroscience 37:171–185
Blomqvist A, Flink R, Bowsher D, Griph S, Westman J (1978) Tectal and thalamic projections of dorsal column and lateral cervical nuclei: a quantitative study in the cat. Brain Res 141:335–341
Blomqvist A, Flink R, Westman J, Wiberg M (1985 a) Synaptic terminals in the ventroposterolateral nucleus of the thalamus from neurones in the dorsal column and lateral cervical nuclei: an electron microscopic study in the cat. J Neurocytol 14:869–886
Blomqvist A, Westman J, Köhler C, Wu J-Y (1985b) Immunocytochemical localization of glutamic acid decarboxylase and substance P in the lateral cervical nucleus: a light and electron microscopic study in the cat. Neurosci Lett 56:229–233
Boivie J (1970) The termination of the cervicothalamic tract in the cat. An experimental study with silver impregnation methods. Brain Res 19:333–360
Boivie J (1980) Thalamic projections from the lateral cervical nucleus in monkey. A degeneration study. Brain Res 198:13–26
Boivie J (1983) Anatomic and physiologic features of the spinocervico-thalamic pathway. In: Macchi G, Rustioni A, Spreafico R (eds) Somatosensory integration in the thalamus. Eisevier, Amsterdam, pp 63–106
Broman J (1994) Neurotransmitters in subcortical somatosensory pathways. Anat Embryol 189:181–214
Broman J, Blomqvist A (1989 a) Substance P-like immunoreactivity in the lateral cervical nucleus of the owl monkey (Aotus trivirgatus): a comparison with the cat and rat. J Comp Neurol 289:111–117
Broman J, Blomqvist A (1989 b) GABA-immunoreactive neurons and terminals in the lateral cervical nucleus of the cynomolgus monkey. J Comp Neurol 283:415–424
Broman J, Blomqvist A (1990) Serotonergic innervation of the lateral cervical nucleus: an immunohistochemical study in cats and monkeys (Aotus trivirgatus). Synapse 6:55–62
Broman J, Ottersen OP (1992) Cervicothalamic tract terminals are enriched in glutamate-like immunoreactivity: an electron microscopic double-labeling study in the cat. J Neurosci 12:204–221
Broman J, Pubols BH Jr (1993) Substance P-like and serotoninlike immunoreactivity in the lateral cervical nucleus of the raccoon. J Comp Neurol 329:354–364
Broman J, Westman J (1988) GABA-immunoreactive neurons and terminals in the lateral cervical nucleus of the cat. J Comp Neurol 274:467–482
Broman J, Westman J, Ottersen OP (1990) Ascending afferents to the lateral cervical nucleus are enriched in glutamate-like immunoreactivity: a combined anterograde transport-immunogold study in the cat. Brain Res 520:178–191
Brown AG (1981) The spinocervical tract. Progr Neurobiol 17:59–96
Bryan RN, Coulter JD, Willis WD (1974) Cells of origin of the spinocervical tract in monkey. Exp Neurol 42:574–586
Cervero F, Iggo A, Molony V (1977) Responses of spinocervical tract neurones to noxious stimulation of the skin. J Physiol (Lond) 267:537–558
Craig AD Jr (1978) Spinal and medullary input to the lateral cervical nucleus. J Comp Neurol 181:729–744
Craig AD Jr, Burton H (1979) The lateral cervical nucleus in the cat: anatomic organization of cervicothalamic neurons. J Comp Neurol 185:329–346
Craig AD Jr, Tapper DN (1978) Lateral cervical nucleus in the cat: functional organization and characteristics. J Neurophysiol 41:1511–1534
Craig AD Jr, Sailer S, Kniffki K-D (1987) Organization of anterogradely labeled spinocervical tract terminations in the lateral cervical nucleus of the cat. J Comp Neurol 263:214–222
Craig AD, Broman J, Blomqvist A (1992) Lamina I spinocervical tract terminations in the medial part of the lateral cervical nucleus in the cat. J Comp Neurol 322:99–110
Downie JW, Ferrington DG, Sorkin LS, Willis WD (1988) The primate spinocervicothalamic pathway: responses of cells in the lateral cervical nucleus and spinocervical tract to innocuous and noxious stimuli. J Neurophysiol 59:861–885
Fedina L, Gordon G, Lundberg A (1968) The source and mechanisms of inhibition in the lateral cervical nucleus of the cat. Brain Res 11:694–696
Flink R, Westman J (1986) Different neuron populations in the feline lateral cervical nucleus: a light and electron microscopic study with the retrograde axonal transport technique. J Comp Neurol 250:265–281
Flink R, Wiberg M, Blomqvist A (1983) The termination in the mesencephalon of fibres from the lateral cervical nucleus. An anatomical study in the cat. Brain Res 259:11–20
Giesler GJ Jr, Elde RP (1985) Immunocytochemical studies of the peptidergic content of fibers and terminals within the lateral spinal and lateral cervical nuclei. J Neurosci 5:1833–1841
Giesler GJ Jr, Urca G, Cannon JT, Liebeskind JC (1979) Response properties of neurons of the lateral cervical nucleus in the rat. J Comp Neurol 186:65–78
Giesler GJ Jr, Björkeland M, Xu Q, Grant G (1988) Organization of the spinocervicothalamic pathway in the rat. J Comp Neurol 268:223–233
Gordon G, Jukes MGM (1963) An investigation of cells in the lateral cervical nucleus of the cat which respond to stimulation of the skin. J Physiol (Lond) 169:28–29
Ha H, Kitai ST, Morin F (1965) The lateral cervical nucleus of the raccoon. Exp Neurol 11:441–450
Hirata H, Pubols BH (1989) Spinocervical tract neurons responsive to light mechanical stimulation of the raccoon forepaw. J Neurophysiol 61:138–148
Holstege JC (1991) Ultrastructural evidence for GABAergic brain stem projections to spinal motoneurons in the rat. J Neurosci 11:159–167
Hongo T, Jankowska E, Lundberg A (1968) Post-synaptic excitation and inhibition from primary afferents in neurones of the spinocervical tract. J Physiol (Lond) 199:569–592
Horrobin DF (1966) The lateral cervical nucleus of the cat; an electrophysiological study. Q J Exp Physiol 51:351–371
Jones BE, Holmes CJ, Rodriguez-Veiga E, Mainville L (1991) GABA-synthesizing neurons in the medulla: their relationship to serotonin-containing and spinally projecting neurons in the rat. J Comp Neurol 313:349–367
Kajander KC, Giesler GJ Jr (1987) Responses of neurons in the lateral cervical nucleus of the cat to noxious cutaneous stimulation. J Neurophysiol 57:1686–1704
Maxwell DJ, Christie WM, Somogyi P (1989) Synaptic connections of GABA-containing boutons in the lateral cervical nucleus of the cat: an ultrastructural study employing pre- and post-embedding immunocytochemical methods. Neuroscience 33:169–184
Millhorn DE, Hökfelt T, Seroogy K, Oertel W, Verhofstad AAJ, Wu J-Y (1987) Immunohistochemical evidence for colocalization of γ-aminobutyric acid and serotonin in neurons of the ventral medulla oblongata projecting to the spinal cord. Brain Res 410:179–185
Oswaldo-Cruz E, Kidd C (1964) Functional properties of neurons in the lateral cervical nucleus of the cat. J Neurophysiol 27:1–14
Ottersen OP (1987) Postembedding light- and electron-microscopic immunocytochemistry of amino acids: description of a new model system allowing identical conditions for specificity testing and tissue processing. Exp Brain Res 69:167–174
Ottersen OP, Storm-Mathisen J, Madsen S, Skumlien S, Strømhaug J (1986) Evaluation of the immunocytochemical method for amino acids. Med Biol 64:147–158
Pubols BH, Haring JH (1995) The raccoon spinocervical and spinothalamic tracts: an HRP study. Brain Res Rev 20:196–208
Rustioni A, Weinberg RJ (1989) The somatosensory system. In: Björklund A, Hökfelt T, Swanson LW (eds) Handbook of chemical neuroanatomy, vol 7. Integrated systems of the CNS, part II. Elsevier, Amsterdam, pp 219–321
Simone DA, Pubols BH (1991) The raccoon lateral cervical nucleus: a single-unit analysis. J Neurophysiol 65:1411–1421
Svensson BA, Rastad J, Westman J, Wiberg M (1985) Somatotopic termination of spinal afferents to the feline lateral cervical nucleus. Exp Brain Res 57:576–584
Westman J (1971) The lateral cervical nucleus in the cat. V. A quantitative evaluation on the bouton- and glia-covered surface area of different LCN-neurons. Z Zellforsch Mikrosk Anat 115:377–387
Wiberg M, Westman J, Blomqvist A (1987) Somatosensory projection to the mesencephalon: an anatomical study in the monkey. J Comp Neurol 264:92–117
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Broman, J., Pubols, B.H. The raccoon lateral cervical nucleus: mediolateral organization of GABA-positive and GABA-negative neurons and fibers. Anat Embryol 193, 463–474 (1996). https://doi.org/10.1007/BF00185877
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00185877