Abstract
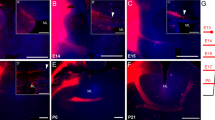
Many immunocytochemical studies have identified different types of neurotransmitters localized in the corpus callosum (CC) axons in the adult mammal. Few studies have looked at the development of different neurochemically identified CC systems. Previous studies on the development of cat CC axons have indicated that a large number of transitory CC axons project to the cortex during early postnatal development. The present study focuses on the development of one neurochemically identified group of CC axons in the cat, labeled with an antibody against neuropeptide Y (NPY), to determine if this group participates in transitory CC axonal growth. Cats at specified ages from birth to adulthood were studied with a routine method of immunocytochemistry for antiserum to NPY. NPY-immunoreactive (ir) CC axons were detected at all stages examined, from newborn to adult; the peak density occurred during postnatal weeks (PNW) 3–4. During PNW 1–2, the denisty of NPY-ir CC axons increased gradually; some NPY-ir axons at this age had growth cones located within the CC bundle between the cerebral hemispheres. The density of the NPY-ir CC axons decreased gradually during PNW 5–7, and from PNW 8 to maturity only a few NPY-ir CC axons were observed. These results indicate that at least two types of NPY-ir CC axons (i.e., transitory and permanent) exist during development, and that most of these axons are eliminated or only express NPY-ir for a short period during development. The results also indicate that neurochemical subsets of CC axons participate in the extensive transitory growth observed by means of the membrane tracer DiI but they may follow unique developmental timetables.
Similar content being viewed by others
References
Antonopoulos J, Papadopoulos GC, Michaloudi H, Cavanagh ME, Parnavelas JG (1992) Postnatal development of NPY-containing neurons in the visual cortex of normal and dark reared rats. Neurosci Lett 145:75–78
Berbel P, Innocenti GM (1988) The development of the corpus callosum in cats: a light- and electron microscopic study. J Comp Neurol 276:132–156
Cavanagh ME, Parnavelas JG (1990) Development of neuropeptide Y (NPY) immunoreactive neurons in the rat occipital cortex: a combined immunohistochemicalautoradiographic study. J Comp Neurol 297:553–563
Chalupa LM, Killackey HP (1989) Process elimination underlies ontogenetic change in the distribution of callosal projection nrurons in the postcentral gyrus of the fetal rhesus monkey. Proc Natl Acad Sci USA 86:1076–1079
Chronwall BM, DiMaggio DA, Massari VJ, Pickel VM, Ruggiero DA, O'Donohue TL (1985) The anatomy of neuropeptide Y containing neurons in the rat brain. Neuroscience 15:1159–1181
Chun JJM, Nakamura MJ, Shatz CJ (1987) Transient cells of the developing telencephalon are peptide-immunoreactive neurons. Nature 325:617–620
Cobas A, Alvarez-Bolado G, Fairen A (1988) Transient GABA-like immunoreactive axons in the corpus callosum of perinatal rats. Neurosci Lett 93:7–12
Conti F, Fabri M, Manzoni T (1988) Glutamate-positive corticocortical neurons in the somatic sensory areas I and II of cats. J Neurosci 8:2948–2960
Demeulemeester H, Vandesande F, Orban GA, Brandon C, Vanderhaeghen JJ (1988) Heterogeneity of the GABAergic cells in cat visual cortex. J Neurosci 8:988–1000
Ding S-L, Elberger AJ (1992) Neuropeptide Y immunoreactivity in cat corpus callosum axons during development. Soc Neurosci Abstr 18:773
Ding S-L, Elberger AJ (1993) Existence of transitory corpus callosum axons throughout visual cortex in neonatal cat and rat confirmed using in vivo biotinylated dextran amine. Soc Neurosci Abstr 19:892
Ding S-L, Zheng DS (1990) Immunocytochemical evidence for glutamate- and GABA-containing callosal neurons in rat. Acta Anat Sinica 21:394–398
Dinopoulos A, Dori I, Davies SW, Parnavelas JG (1989) Neurochemical heterogeneity among corticofugal and callosal projections. Exp Neurol 105:36–44
Elberger AJ (1979) The role of the corpus callosum in the development of interocular eye alignment and the organization of the visual field in the cat. Exp Brain Res 36:71–85
Elberger AJ (1981) Ocular dominance in striate cortex is altered by neonatal section of the posterior corpus callosum in the cat. Exp Brain Res 41:280–291
Elberger AJ (1982) The corpus callosum is a critical factor for developing maximum visual acuity. Dev Brain Res 5:350–353
Elberger AJ (1984) The existence of a separate, brief critical period for the corpus callosum to affect visual development. Behav Brain Res 11:223–231
Elberger AJ (1988) Critical role of the corpus callosum in the visual development of cats. In: Petit TL, Ivy GO (eds) Neural plasticity: a lifespan approach. Liss, New York, pp 105–123
Elberger AJ (1989a) Binocularity and single cell acuity are related in striate cortex of corpus callosum sectioned and normal cats. Exp Brain Res 77:213–216
Elberger AJ (1989b) Selective labeling of visual corpus callosum connections with aspartate in cat and rat. Visual Neurosci 2:81–85
Elberger AJ (1990) Spatial frequency thresholds of single striate cortical cell in neonatal corpus callosum sectioned cats. Exp Brain Res 82:617–627
Elberger AJ (1993) Distribution of transitory corpus callosum axons projecting to developing cat visual cortex revealed by DiI. J Comp Neurol 333:326–342
Elberger AJ (1994) Transitory corpus callosum axons projecting to developing rat visual cortex revealed by DiI. Cereb Cortex (in press)
Elberger AJ, Honig MG (1990) Double-labeling of tissue containing the carbocyanine dye DiI for immunocytochemistry. J Histochem Cytochem 38:735–739
Elberger AJ, Smith III EL (1985) The critical period for corpus callosum section to affect cortical binocularity. Exp Brain Res 57:213–223
Elberger AJ, Hester MM, Ding S-L (1992) Transitory corpus callosum axon terminals in rat show synapse formation in visual cortex. Soc Neurosci Abstr 18:1305
Feng JZ, Brugge JF (1983) Postnatal development of auditory callosal connections in the kitten. J Comp Neurol 214:416–426
Fleischhauer K, Wartenberg H (1967) Elektronenmikroskopische Untersuchungen über das Wachstum der Nervenfasern und über das Auftreten von Markscheiden im Corpus callosum der Katze. Z Zellforsch Mikrosk Anat 83:568–581
Foster GA, Schultzberg M (1984) Immunohistochemical analysis of the ontogeny of neuropeptide Y immunoreactive neurons in the fetal rat brain. Int J Dev Neurosci 2:387–407
Hogan D, Berman NEJ (1992) The development of neuropeptide Y immunoreactive neurons in cat visual cortical areas. Dev Brain Res 67:343–369
Hughes CM, Peters A (1990) Morphological evidence for callosally projecting nonpyramidal neurons in rat visual cortex. Anat Embryol 182:591–603
Innocenti GM (1981) Growth and reshaping of axons in the establishment of visual callosal connections. Science 212:824–827
Innocenti GM, Caminiti R (1980) Postnatal shaping of callosal connections from sensory areas. Exp Brain Res 38:381–394
Innocenti GM, Clarke S (1984) The organization of immature callosal connections. J Comp Neurol 230:287–309
Innocenti GM, Clarke S, Kraftsik (1986) Interchange of callosal and association projections in the developing visual cortex. J Neurosci 6:1384–1409
Ivy GO, Killackey HP (1981) The ontogeny of the distribution of callosal projection neurons in the rat parietal cortex. J Comp Neurol 195:367–389
Ivy GO, Killackey HP (1982) Ontogenetic changes in the projections of neocortical neurons. J Neurosci 2:735–743
Jones EG, Hendry SHC (1986) Co-localization of GABA and peptides in neocortical neurons. Trends Neurosci 9:71–76
LaMantia A-S, Rakic P (1990) Axon overproduction and elimination in the corpus callosum of the developing rhesus monkey. J Neurosci 10:2156–2175
Looney GA, Elberger AJ (1986) Myelination of the corpus callosum in the cat: time course, topography, and functional implications. J Comp Neurol 248:336–347
Nakagawa Y, Shiosaka S, Emson PC, Tohyama M (1985) Distribution of neuropeptide Y in the forebrain and diencephalon: an immunohistochemical analysis. Brain Res 361:52–60
Norris CR, Kalil K (1990) Morphology and cellular interactions of growth cones in the developing corpus callosum. J Comp Neurol 293:268–281
Olavarria J, Van Sluyters RC (1985) Organization and postnatal development of callosal connections in the visual cortex of the rat. J Comp Neurol 239:1–26
O'Leary DDM, Stanfield BB, Cowan WM (1981) Evidence that the early postnatal restriction of the cells of origin of the callosal projections is due to the elimination of axonal collaterals rather than to the death of neurons. Dev Brain Res 1:607–617
Parnavelas JG, Cavanagh ME (1988) Transient expression of neurotransmitters in the developing neocortex. Trends Neurosci 11:92–93
Peters A, Payne BR, Josephson K (1990) Transcallosal nonpyramidal cell projections from visual cortex in the cat. J Comp Neurol 302:124–142
Seroogy KB, Fallon JH, Loughlin SE, Leslie FM (1985) Few cortical CCK immunoreactive neurons have long projections. Exp Brain Res 59:533–542
Shatz CJ, Chun JJM, Luskin MB (1988) The role of the subplate in the development of the mammalian telencephalon. In: Peters A, Jones EG (eds) Cerebral cortex, vol 7. Plenum Press, New York, pp 35–58
Tigges M, Tigges J, McDonald JK, Slattery M, Fernandes A (1989) Postnatal development of neuropeptide Y-like immunoreactivity in area 17 of normal visually deprived rhesus monkeys. Vis Neurosci 2:315–328
Tsumoto T (1990) Excitatory amino acid transmitters and their receptors in neural circuits of the cerebral neocortex. Neurosci Res 9:79–102
Van Reeth O, Goldman S, Schiffmann S, Verstappen A, Pelletier G, Haudry H, Vanderhaeghen JJ (1987) Distribution of neuropeptide Y immunoreactivity in human visual cortex and underlying white matter. Peptides 8:1107–1117
Voigt T, LeVay S, Stamnes M (1988) Morphological and immunocytochemical observations on the visual callosal projectons in the cat. J Comp Neurol 272:450–460
Wahle P, Meyer GJ (1987) Morphology and quantitative changes of transient NPY-ir neuronal populations during early postnatal development of the cat visual cortex. J Comp Neurol 261:165–192
Wahle P, Meyer GJ, Albus K (1986) Localization of NPY-immunoreactivity in the cat's visual cortex. Exp Brain Res 61:364–374
Wahle P, Meyer GJ, Wu J-Y, Albus K (1987) Morphology and axon terminal pattern of glutamate decarboxylase-immunoreactive cell types in the white matter of the cat occipital cortex during early postnatal development. Dev Brain Res 36:53–61
Woodhams PL, Allen YS, McGovern J, Allen JM, Bloom SR, Balazs R, Polak JM (1985) Immunohistochemical analysis of the early ontogeny of the neuropeptide Y system in the rat brain. Neuroscience 15:173–202
Yamashita A, Shimizu K, Hayashi M (1990) Ontogeny of substance P-immunoreactive structures in the primate cerebral cortex. Dev Brain Res 57:197–207
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ding, SL., Elberger, A.J. Neuropeptide Y immunoreactive axons in the corpus callosum of the cat during postnatal development. Anat Embryol 190, 55–63 (1994). https://doi.org/10.1007/BF00185846
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00185846