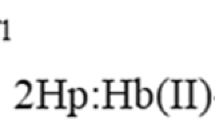Abstract
Kinetics of the reconstitution of hemoglobin from semihemoglobins α and β with hemin dicyanide have been investigated using three kinds of stopped-flow technique (Soret absorption, fluorescence quenching of tryptophan, and Soret CD). The semihemoglobins α and β are occupied by heme in the α and β chains, respectively, the other chain being heme-free. Based on the kinetic results, the following scheme for the reconstitution is proposed; First, hemin dicyanide enters the pocket-like site of the apo chains. Second, in semihemoglobin α, the CN-ligand in the fifth coordination position of iron is replaced by the imidazole ring of the proximal His immediately after the heme insertion. In contrast, semihemoglobin β changes its conformation after the heme insertion, and this is followed by the ligand replacement. Finally, the partial structure changes induced by the ligand replacement propagate onto the whole molecule and the final conformation is attained. The results indicate that semihemoglobin α retains a more rigid and organized structure, and more closely approaches its final structure than does semihemoglobin β.
Similar content being viewed by others
References
Aojula HS, Wilson MT, Drake A (1986) Characterization of haem disorder by circular dichroism. Biochem J 237:613–616
Bunn HF, Forget BG, Ranney HM (1977) Human Hemoglobin: Coordination of α- and β-chain synthesis and assembly of hemoglobin tetramers. Saunders, Philadelphia, pp 126–131
Cannon JB, Kuo F-S, Pasternack RF, Wrong NM, Muller-Eberhard U (1984) Kinetics of the interaction of hemin liposomes with heme binding proteins. Biochemistry 23:3715–3721
Cassoly R (1981) Preparation of globin-hemoglobin hybrids: Artificially prepared and naturally occurring semihemoglobins. Methods Enzymol 76:121–125
Chu AH, Bucci E (1979) Effect of polyanions on the kinetics of the reaction of apohemoglobin with carbonmonoxy heme. J Biol Chem 254:3772–3776
Gibson QH, Antonini E (1963) Rates of reaction of native human globin with some hemes. J Biol Chem 238:1384–1388
Hochstrasser RM, Negus DK (1984) Picosecond fluorescence decay of tryptophans in myoglobin. Proc Natl Acad Sci USA 81:4399–4403
Hsu M-C, Woody RW (1971) The origin of the heme cotton effects in myoglobin and hemoglobin. J Am Chem Soc 93:3515–3525
Ishimori K, Morishima I (1988) Study of the specific heme orientation in reconstituted hemoglobins. Biochemistry 27:4747–4753
Kawamura-Konishi Y, Suzuki H (1985) Binding reaction of hemin to globin. J Biochem 98:1181–1190
Kawamura-Konishi Y, Kihara H, Suzuki H (1988) Reconstitution of myoglobin from apoprotein and heme, monitored by stopped-flow absorption, fluorescence and circular dichroism. Eur J Biochem 170:589–595
Kawamura Y, Hasumi H, Nakamura S (1982) Kinetic studies on the reconstitution of deoxyhemoglobin from isolated α and β chains. J Biochem 92:1227–1233
Kawamura Y, Nakamura S (1983) Assembly of oxyhemoglobin from isolated α and β chains. J Biochem 93:1159–1166
La Mar GN, Davis NL, Parish DW, Smith KM (1983) Heme orientational disorder in reconstituted and native sperm whale myoglobin. J Mol Biol 168:887–896
La Mar GN, Toi H, Krishnamoorthi R (1984) Proton NMR investigation of the rate and mechanism of heme rotation in sperm whale myoglobin: Evidence for intramolecular reorientation about a heme twofold axis. J Am Chem Soc 106:6395–6401
Leutzinger Y, Beychok S (1981) Kinetics and mechanism of heme-induced refolding of human α-globin. Proc Natl Aca Sci USA 78:780–784
Light WR, Rohlfs RJ, Palmer G, Olson JS (1987) Functional effects of heme orientational disorder in sperm whale myoglobin. J Biol Chem 262:46–52
Lim VI, Efimov AV (1977) The folding pathway for globins, FEBS Lett 78:279–283
Mawatari K, Matsukawa S, Yoneyama Y (1983) Different effects of subunit association upon absorption and circular dichroism spectra of methemoglobin. Biochim Biophys Acta 745:219–228
Nelder JA, Mead R (1965) A simplex method for function minimization. Comput J 7:308–313
Park RY, McDonald MJ (1989) Kinetics of heme binding to semi-alpha-hemoglobin. Biochim Biophys Res Commun 162:522–527
Perutz MF, Muirhead H, Cox JM, Goaman LCG (1968) Three-dimensional fourier synthesis of horse oxyhaemoglobin at 2.8 Å resolution: The atomic model. Nature 219:131–139
Perutz MF (1987) The molecular basis of blood diseases: Molecular anatomy, physiology, and pathology of hemoglobin. Saunders, Philadelphia, pp 127–178
Riggs A (1981) Preparation of blood hemoglobins of vertebrates. Methods Enzymol 76:5–29
Rose MY, Olson JS (1983) The kinetic mechanism of heme binding to human apohemoglobin. J Biol Chem 258:4298–4303
Shack J, Clark WM (1947) Metalloporphyrins VI. Cycles of changes in systems containing heme. J Biol Chem 171:143–187
Yamamoto Y, La Mar GN (1986) 1H NMR study of dynamics and thermodynamics of heme rotational disorder in native and reconstituted hemoglobin A. Biochemistry 25:5288–5297
Yip YK, Waks M, Beychok S (1977) Reconstitution of native human hemoglobin from separated globin chains and alloplex intermediates. Proc Natl Acad Sci USA 74:64–68
Yoshikawa S, O'Keeffe DH, Caughey WS (1985) Investigations of cyanide as an infrared probe of hemeprotein ligand binding sites. J Biol Chem 260:3518–3528
Author information
Authors and Affiliations
Additional information
Correspondence to: Y. Kawamura-Konishi
Rights and permissions
About this article
Cite this article
Kawamura-Konishi, Y., Chiba, K., Kihara, H. et al. Kinetics of the reconstitution of hemoglobin from semihemoglobins α and β with heme. Eur Biophys J 21, 85–92 (1992). https://doi.org/10.1007/BF00185423
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00185423