Abstract
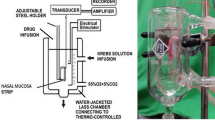
In thiopentone-anesthetized mature pigs (n = 7), local intra-arterial infusion of the β2-adrenoceptor agonists terbutaline and salbutamol and the β3-agonist BRL 37344 induced dose-dependent increases in nasal arterial blood flow (BF) and volume of the nasal mucosa (reflecting capacitance vessel function). Increases were also found in the laser Doppler flowmeter signal, reflecting superficial mucosal BE. In contrast to terbutaline and salbutamol, BRL 37344 showed marked effects on volume. Pretreatment with the β-adrenoceptor blocker propranolol significantly reduced the vasodilatory effects of terbutaline and salbutamol, whereas the BF increase evoked by BRL 37344 was not affected. Exogenous noradrenaline (NA) induced in vitro dose-dependent contractions of human nasal mucosa biopsies obtained from patients with non-allergic chronic rhinitis (n = 21) or non-allergic nasal polyposis (NANP, n = 16). On a molar basis, the contractile effect of NA was significantly greater in nasal mucosa samples without histological abnormalities when compared to biopsies with abundant inflammatory cells and edema within the submucosa. In the presence of propranolol, the vasoconstrictor effect of NA was significantly enhanced in biopsies with abundant inflammatory cells obtained from patients with NANP (P<0.01). This observation suggests the possible occurrence of a β2 hyper-reactivity in the nasal mucosa of patients with NANP. After precontraction in a Krebs-Ringer solution with 50 nM K+, all nasal biopsies studied showed dose-dependent relaxation to terbutaline, salbutamol and BRL 37344. This relaxant effect was markedly reduced after pretreatment with propranolol. These observations suggest that β2- and β3-adrenergic vasodilatatory mechanisms may be similar in the nasal mucosa of the pig and man.
Similar content being viewed by others
References
Andersson KE, Bende M (1984) Adrenoceptors in the control of human nasal mucosal blood flow. Ann Otol Rhinol Laryngol 93:179–182
Ängg»rd A (1974) Capillary and shunt blood flow in feline nasal mucosa. Acta Otolaryngol (Stockh) 78:418–422
Ängg»rd A, Densert O (1974) Adrenergic innervation of the nasal mucosa in cat. Acta Otolaryngol (Stockh) 78:232–241
Arch JRS, Ainsworth AT, Cawthorne MA, Piercy V, Sennit MA, Thody V, Wilson C, Wilson S (1984) Atypical betaadrenoceptor on brown adipocytes as a target for antiobesity drugs. Nature 309: 163–165
Bumsted RM, El-Ackad T, Montgomery S, Brody MJ (1979) Histamine, norepinephrine and serotonin content of nasal polyps. Laryngoscope 89:832–843
Dahlström A, Fuxe K (1965) The adrenergic innervation of the nasal mucosa of certain animals. Acta Otorhinolaryngol (Stockh) 59:65–72
Emorine LJ, Marullo S, Briend-Sutren MM, Patey G, Tate K, Delavier-Klutchko C, Strosberg D (1989) Molecular characterisation of the human beta-3 adrenergic receptor. Science 245 1118–1121
Euler US von (1948) Identification of sympathomimetic ergone in adrenergic nerves of cattle (Sympathetin N) with laevo-noradrenaline. Acta Physiol Scand 16:63–74
Holloway BR, Howe R, Rao BS, Stribling D (1989) ICI 198127: a novel selective agonist of brown fat and thermogenesis. In: Bjorntorp P, Rossner S (eds) Obesity in Europe 88. Libbey, London, pp 323–328
Jackson RT (1979) A new in vitro method of drug assay of nasal blood vessels. Arch Otorhinolaryngol 225:33–38
Lacroix JS (1989) Adrenergic and non-adrenergic mechanisms in the sympathetic vascular control of the nasal mucosa. Acta Physiol Scand 136 [Suppl 582]:1–67
Lacroix JS, Lundberg JM (1989) Sympathetic vascular control of the pig nasal mucosa: adrenoceptor mechanisms in blood flow and volume control. Br J Pharmacol 970:1075–1084
Malm L (1974) β-adrenergic receptors in the vessels of the cat nasal mucosa. Acta Otolaryngol (Stockh) 78:242–246
Pierce J, Suelter CH (1977) An evaluation of the Coomassie brillant blue G-250 dye-binding method for quantitative protein determination. Anal Biochem 82:478–480
Shelhamer JH, Metcalfe DD, Smith LJ, Kaliner M (1980) Abnormal beta adrenergic responsiveness in allergic subjects: analysis of isoproterenol-induced cardiovascular and plasma cyclic adenosine monophosphate response. J Allergy Clin Immunol 66:52–60
Starke K (1981) α-adrenoceptor subclassification. Rev Physiol Biochem Pharmacol 88:199–236
Starke K (1987) Pre-synaptic alpha-autoreceptors. Rev Physiol Biochem Pharmacol 107:146–173
Wilson C, Wilson S, Piercy V, Sennit MV, Arch IRS (1984) The rat lipolytic β-adrenoceptor: studies using novelß-adreno-ceptor agonist. Eur J Pharmacol 100: 309–319
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Lacroix, J.S., Kurt, A.M., Auberson, S. et al. Beta-adrenergic mechanisms in the nasal mucosa vascular bed. Eur Arch Otorhinolaryngol 252, 298–303 (1995). https://doi.org/10.1007/BF00185393
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00185393