Abstract
• Background: Ocular pulse amplitude (OPA) was measured to investigate the influence of peripheral vasoconstriction and vasodilatation on choroidal perfusion in healthy volunteers and to determine whether low OPA in low-tension glaucoma (LTG) patients is associated with a vasospastic reaction and its response to the calcium channel blocker nifedipine.
• Methods: OPA was determined using the Langham ocular blood flow (OBF) system, applanation intraocular pressure (IOP), systemic blood pressure, and heart rate were measured, and ocular perfusion pressure was calculated before and after exercise and smoking in 12 otherwise nonsmoking, healthy volunteers and prior to and for 3 months after initiation of nifedipine therapy in 32 LTG patients with and without a vasospastic reaction as manifested by a nailfold capillary blood flow test.
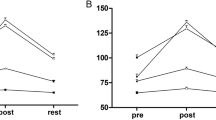
• Results: Exercise significantly (P<0.05) increased heart rate, systolic blood pressure and ocular perfusion pressure, while it significantly (P<0.05) reduced IOP and diastolic blood pressure. However, OPA was not significantly (P>0.1) affected by changes in these parameters. Smoking significantly (P<0.05) increased systolic and diastolic blood pressure, heart rate, and ocular perfusion pressure but did not significantly (P>0.09) alter OPA. There were two distinct LTG subtypes, with and without a vasospastic reaction. Only those with a vasospastic reaction showed a significant (P<0.001) increase in OPA after 3 months of nifedipine treatment, while all other parameters tested were not significantly altered.
• Conclusion: Despite affecting ocular and systemic perfusion parameters, exercise and smoking did not alter OPA, suggesting functional isolation, i.e. autoregulation of the choroidal and/or ophthalmic artery circulation in healthy volunteers. Low OPA in LTG was increased by nifedipine only in vasospastic LTG patients, suggesting different, vasotonus-related pathologies. Calcium channel blockers and other vasodilators may be useful in vasoreactive LTG patients with reduced OPA.
Similar content being viewed by others
References
Alm A, Bill A (1972) The oxygen supply to the retina. II. Effects of high intraocular pressure and increased arterial carbon dioxide tension on uveal and retinal blood flow in cats. Acta Physiol Scand 84: 306–319
Alm A, Bill A (1973) Ocular and optic nerve blood flow at normal and increased intraocular pressures in monkeys (Macaca iris): a study with radioactively labelled microspheres including flow determinations in brain and some other tissues. Exp Eye Res 15: 15–29
Alm A, Bill A, Young FA (1973) The effects of pilocarpine and neostigmine on the ocular blood flow through the anterior uvea in monkeys. A study with radioactively labelled microspheres. Exp Eye Res 15: 31–36
Bill A (1975) Blood circulation and fluid dynamics in the eye. Physiol Rev 55: 383
Bill A (1981) Ocular circulation. In: Moses RA (ed) Adler's physiology of the eye. Mosby, St. Louis, pp 184–203
Bill A, Sperber GO (1989) Control of retinal and choroidal blood flow. Eye 4: 319–325
Drance SM, Douglas GR, Wijsman K, Schulzer M, Britton RJ (1988) Response of blood flow to warm and cold in normal and low tension glaucoma. Am J Ophtalmol 105:35–39
Forest CR, Xu N, Pang CY (1994) Evidence for nicotine induced skin flap ischemic necrosis in the pig. Can J Physiol Pharmacol 72: 30–38
Friedmann E, Kopald H, Smith T (1964) Retinal and choroidal blood flow determined with krypton-85 in anesthesized animals. Invest Ophthalmol Vis Sci 3: 539–547
Hales JR, Ludbrook J (1988) Baroreflex participation in redistribution of cardiac output at onset of exercise. J Appl Physiol 64: 627–634
Harris A, Malinovsky V, Martin B (1994) Correlates of acute exercise-induced ocular hypotension. Invest Ophthalmol Vis Sci 35: 3852–3857
Harris A, Sergott RC, Spaeth GL, Katz JL, Shoemaker JA, Martin BJ (1994) Color doppler analysis of ocular vessel blood velocity in normal-tension glaucoma. Am J Ophthalmol 118:642–649
Kiowski W, Linder L, Stoschitzky K, Pfisterer M, Burckhardt D, Burkart F, Buhler RF (1994) Diminished vascular response to inhibition of endothelium-derived administered endothelin-1 in clinically healthy smokers. Circulation 90: 27–34
Kitazawa Y, Shirai H, Go FJ (1989) The effect of a Ca++ antagonist on visual field in low tension glaucoma. Graefe's Arch Clin Exp Ophthalmol 227:408–412
Lumme P, Tuulonen A, Airaksinen PJ, Alanko HI (1991) Neuroretinal rim area in low tension glaucoma: effect of nifedipine and acetazolamide compared to no treatment. Acta Ophthalmol Copenh 69: 293–298
Mahler F, Saner H, Boss C, et al (1987) Local cold exposure test for capillaroscopic examination of patients with Raynaud's syndrome. Microvasc Res 33:422–427
Marcus DF, Krupin T, Podos SM, Becker B (1970) The effect of exercise on intraocular pressure. I. Human beings. Invest Ophthalmol 9: 749–752
Mittag TW, Serle J, Schumer R, Brodie S, Stegmann D, Schmidt KG, Taniguchi T, Henn Rho Sae, Podos SM (1994) Studies of the ocular pulse in primates. Surv Ophthalmol 38 [Suppl]: 183–190
Netland PA, Chaturvedi N, Dreyer EB (1993) Calcium channel blockers in the management of low tension and open-angle glaucoma. Am J Ophthalmol 115: 608–613
Parver LM (1991) Temperature modulating action of choroidal blood flow. Eye 5: 181–185
Phelps CD, Corbett JJ (1985) Migraine and low tension glaucoma. A case control study. Invest Ophthalmol Vis Sci 26: 1105–1108
Pillunat LE, Stodtmeister R, Marquardt R, Mattern A (1989) Ocular perfusion pressures in different types of glaucoma. Int Ophthalmol 13: 37–42
Quigley HA, Hohmann RM, Addicks EM, Green WR (1984) Blood vessels of the glaucomatous optic disc in experimental primate and human eyes. Invest Ophthalmol Vis Sci 25: 918–931
Rojanapongpun P, Drance SM, Morrison BJ (1993) Ophthalmic artery flow velocity in glaucomatous and normal subjects. Br J Ophthalmol 77: 25–29
Schmidt KG, Stegman DY, Serle JB, Garrett DT, Camras CB, Mittag TW, Podos SM (1991) Ocular pulse amplitude (OPA) in primary open angle glaucoma, low tension glaucoma, and in ocular hypertensive patients before and after topical drug treatment (ARVO abstract). Invest Ophthalmol Vis Sci 32 [Suppl]: 943
Schwartz B (1986) Changes in optic disc in ocular hypertension and glaucoma. Jpn J Ophthalmol 30: 143–153
Tornquist P, Alm A (1979) Retinal and choroidal contribution to retinal metabolism in vivo. A study in pigs. Acta Physiol Scand 106: 351–357
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Schmidt, KG., Mittag, T.W., Pavlovic, S. et al. Influence of physical exercise and nifedipine on ocular pulse amplitude. Graefe's Arch Clin Exp Ophthalmol 234, 527–532 (1996). https://doi.org/10.1007/BF00184863
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00184863