Abstract
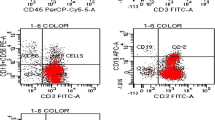
The surface antigens of monocytic ceps m oroncnoalveolar lavage (BAL) fluid were analyzed in 10 patients with sarcoidosis, 8 patients with idiopathic pulmonary fibrosis (IPF), 9 patients with extrinsic allergic alveolitis (EAA), and 10 healthy volunteers, and compared with the surface antigens of peripheral blood monocytes (PBM) of the same individuals.
The absolute numbers of alveolar macrophages (AM) were increased in all disease groups as were the numbers of small monocyte-like cells, indicating an increased influx of PBM into the alveoli, which was the most prominent in EAA patients.
In all groups investigated, the percentages of PBM positive for the monoclonal antibodies (mAb) CD13, CD14, CD33, U26, and Max3 were higher than the percentages of BAL macrophages positive for these markers, while the Max24 marker was equally expressed. In all groups the percentages of AM positive for RFD9 and CD68 were higher than the percentages positive for PBM. The absolute numbers of CD13+ macrophages were increased in IPF and EAA patients, probably due to the increased influx of monocytic cells.
The 3 mAb in the CD68 cluster (i.e., Ki-M6, Ki-M7, and Y2/131) demonstrated marked differences in expression on PBM as well as on AM. This is probably because CD68(Ki-M6) recognizes a different epitope than CD68(Ki-M7) and CD68(Y2/131). The latter 2 become increasingly expressed by AM and this is paralleled by an increased CD68(KiM6) expression. The expression of CD68, which is associated with the generation of oxygen radicals during the respiratory burst and increased chemiluminescence, tended to be elevated on PBM and AM of IPF patients, although with a broad range.
Similar content being viewed by others
References
Akiyama J-I, Chida K. Sato A. Yamashita A (1988) Four monoclonal antibodies, AMH-1,-2, -3, and -4, give varied reactivities with monocytes, alveolar macrophages, and epitheloid-cell granulomas. J Clin Immunol 8:372–380
Andreesen R, Bross KJ, Osterholz J, Emmrich F (1986) Human macrophage maturation and heterogeneity: analysis with a newly generated set of monoclonal antibodies to differentiation antigens. Blood 67:1257–1264
Bierer BE, Golan DE, Brown CS, Herrmann SH, Burakoff SJ (1989) A monoclonal antibody to LFA-3, the CD2 ligand, specifically immobilizes major histocompatibility comples proteins. Eur J Immunol 19:661–665
Biondi A. Rossing TH, Bennett J, Todd RF III (1984) Surface membrane heterogeneity among human mononuclear phagocytes. J Immunol 132:1237–1243
Bitterman PB, Saltzman LE, Adelberg S, Ferrans VJ, Crystal RG (1984) Alveolar macrophage replication: one mechanism for the expansion of the mononuclear phagocyte population in the chronically inflamed lung. J Clin Invest 74:460–469
Blussé van Oud Alblas A, Van der Linden-Schrever B, R van Furth (1981) Origin and kinetics of pulmonary macrophages during an inflammatory reaction induced by intravenous administration of heat-killed bacillus Calmette-Guérin. J Exp Med 154:235–252
Cabanas C, Sanchez-Madrid F, Bellon E, Figdor CG, Te Velde AA, Fernandez JM, Acevedo A, Bernabeu C (1989) Characterization of a novel myeloid antigen regulated during differentiation of monocytic cells. Eur J Immunol 19:1373–1378
Clement A, Chadelat K, Masliah J, Housset B, Sardet A, Grimfeld A, Tournier G (1987) A controlled study of oxygen metabolite release by alveolar macrophages from children with interstitial lung disease. Am Rev Respir Dis 136:1424–1428
Cordell J, Simmons D, Micklem K, Stross P, Mason DY (1989) The expression of panmacrophage antigens in transfected cells. Tissue Antigens 33:205
Costabel U (1988) The alveolitis of hypersensitivity pneumonitis. Eur Respir J 1:5–9
Emmrich H, Andreesen R (1985) Monoclonal antibodies against differentiation antigens on human macrophages. Immunol Lett 9:321–324
Gant VA, Hamblin AS (1985) Human bronchoalveolar macrophage heterogeneity demonstrated by histochemistry, surface markers and phagocytosis. Clin Exp Immunol 60:539–545
Goyert SM, Ferrero EM, Seremetis SV, Winchester RJ, Silver J, Mattison AC (1986) Biochemistry and expression of myelomonocytic antigens. J Immunol 137:3909–3914
Goyert SM, Ferrero E, Rettig WJ, Yenamandra AK, Obata F, Le Beau MM (1988) The CD14 monocyte differentiation antigen maps to a region encoding growth factors and receptors. Science 239:497–499
Griffin JD, Mayer RJ, Weinstein HJ, Rosenthal DS, Coral FS, Beveridge RP, Schlossman SF (1983) Surface marker analysis of acute myeloblastic leukemia: identification of differentiation-associated phenotypes. Blood 62:557–563
Griffin JD, Ritz J, Nadler LM, Schlossman SF (1981) Expression of myeloid differentiation antigens on normal and malignant myeloid cells. J Clin Invest 68:932–941
Hance AJ, Douches S, Winchester RJ, Ferrans VJ, Crystal RG (1985) Characterization of mononuclear phagocyte subpopulations in the human lung by using monoclonal antibodies: changes in alveolar macrophage phenotype associated with pulmonary sarcoidosis. J Immunol 134:284–292
Harvey J, Jones DB, Wright DH (1990) Differential expression of MHC- and macrophage-associated antigens in human fetal and postnatal small intestine. Immunology 69:409–415
Hogg N, Selvendran Y (1985) An anti-human monocyte/macrophage monoclonal antibody, reacting most strongly with macrophages in lymphoid tissue. Cell Immunol 92:247–253
Hogg N, Macdonald S, Slusarenko M, Beverley PCL (1984) Monoclonal antibodies specific for human monocytes, granulocytes and endothelium. Immunology 53:753–767
Johnston RB (1988) Current concepts in immunology monocytes and macrophages. N Engl J Med 318:747–752
Kiemle-Kallee J, Kreipe H, Radzun HJ, Parwaresch MR, Auerswald U, Magnussen H, Barth J (1991) Alveolar macrophages in idiopathic pulmonary fibrosis display a more monocyte-like immunophenotype and an increased release of free oxygen radicals. Eur Respir J 4:400–406
Kreipe H, Radzun HJ, Parwaresch MR, Haislip A, Hansmann M-L (1987) Ki-M7 monoclonal antibody specific for myelomonocytic cell lineage and macrophages in human. J Histochem Cytochem 35:1117–1126
Lo SK, van Seventer GA, Levin SM, Wright SD (1989) Two leukocyte receptors (CDIIa/CD18 and CDIIb/CD18) mediate transient adhesion to endothelium by binding to different ligands. J Immunol 143:3325–3329
Look AT, Ashmun RA, Shapiro LH, Peiper SC (1989) Human myeloid plasma membrane glycoprotein CD13 (gp150) is identical to aminopeptidase. J Clin Invest 83:1299–1307
Macintyre EA, Jones HM, Roberts PJ, Tidman N, Linch DC (1987) Identification of signal transduction molecules on monocytic cells. In: McMichael A (ed) Leucocyte typing III, Oxford University Press, Oxford, pp 685–688
Mayernik DG, UI-Haq A, Rinehart JJ (1983) Differentiation-associated alteration in human monocyte-macrophage accessory cell function. J Immunol 130:2156–2160
Mechtersheimer G, Möller P (1989) Phenotyping of human epithelioid cells, alveolar macrophages, and foamy cells by means of mAb of the myeloid section. In: Knapp W, Dörken B, Gilkes WR, Rieber EP, Schmidt RE, Stein H, Von dem Borne AEGKr (eds) Leucocyte typing IV. Oxford University Press, Oxford, pp 918–921
Micklem K, Rigney E, Pulford K, Turley H, Mason DY (1989) Four pan-macrophage antibodies from the third workshop recognise the same antigen. Tissue Antigens 33:206
Moore ME, Rossman MD, Schreiber AD, Douglas SD (1983) DR framework antigens and 63D3 antigens on human peripheral blood monocytes and alveolar macrophages. Clin Immunol Immunpathol 29:119–128
Noble B, Du Bois RM, LW Poulter (1989) The distribution of phenotypically distinct macrophage subsets in the lungs of patients with cryptogenic fibrosing alveolitis. Clin Exp Immunol 76:41–46
Oliver AM (1990) Macrophage heterogeneity in human fetal tissue. Clin Exp Immunol 80:454–459
Parwaresch MR, Radzun HJ, Kreipe H, Hansmann M-L, Barth J (1986) Monocyte/macrophage-reactive monoclonal antibody Ki-M6 recognizes an intracytoplasmic antigen. Am J Pathol 124:141–151
Peiper SC, LeBoeuf RD, Prasthofer EF, Borowitz MJ, Walker WS, Ashmun RA, Look AT (1989) Biochemical and genetic characterization of gp67, the CD33 myeloid antigen. Tissue Antigens 33:229
Radzun HJ, Parwaresch MR, Bödewadt S, Sundström C, Lennert K (1985) Bimodal differentiation prospectives for promyelocytes. J Natl Cancer Inst 75:199–206
Radzun H, Parwaresch MR, Kreipe H (1983) Monocytic origin of human alveolar macrophages. J Histochem Cytochem 31:318–324
Seymour GJ, Poulter LW, Bofill M, Hobbs S, Janossy G, Brooks D, Zola H, Bradley J (1983) The reactivity of a monoclonal antibody against cells of the monocyte-macrophage series in sections of normal and inflamed human tissues. J Reticuloendothel Soc 34:463–473
Shellito J, Esparza C, Armstrong C (1987) Maintenance of the normal rat alveolar macrophage cell population: The roles of monocyte influx and alveolar macrophage proliferation in situ. Am Rev Respir Dis 135:78–82
Snider GL (1988) Interstitial lung disease: pathogenesis, pathophysiology, and clinical presentation. In: Schwarz MI, King RE (eds) Interstitial lung disease. BC Decker, Toronto, pp 1–15
Sunder-Plassmann G, Wagner L, Apperl A, Hruby K, Balcke P (1989) Modulation of neutrophil respiratory burst and chemotaxis by the myeloid panel mAb. In: Knapp W, Dörken B, Gilkes WR, Rieber EP, Schmidt RE, Stein H, von dem Borne AEGKr (eds) Leucocyte Typing IV. Oxford University Press, Oxford, pp 896–899
Turner-Warwick M (1988) Infiltrative and interstitial lung diseases: General principles and diagnostic approach. In: Murray JF, Nadel JA (eds) Textbook of respiratory medicine. WB Saunders, Philadelphia, pp 1435–1444
Van der Schoot CE, Huizinga TWJ, Gadd S, Majdic O, Wijmans R, Knapp W, von dem Borne AEGKr (1989) Identification of three novel PI-liked proteins on granulocytes. In: Knapp W, Dörken B, Gilkes WR, Rieber EP, Schmidt RE, Stein H, von dem Borne AEGKr (eds) Leucocyte Typing IV. Oxford University Press, Oxford, pp 887–891
Van Dongen JJM, Adriaansen HJ, Hooijkaas H (1988) Immunophenotyping of leukaemias and non-Hodgkin's lymphomas Immunological markers and their CD codes. Neth J Med 33:298–314
Wallis WJ, Beatty PG, Ochs HD, Harlan JM (1985) Human monocyte adherence to cultured vascular endothelium: monoclonal antibody-defined mechanisms. J Immunol 135:2323–2330
Zwadlo G, Bröcker E-B, Von Basseqitz D-B, Feige U, Sorg C (1985) A monoclonal antibody to a differentiation antigen present on mature human macrophages and absent from monocytes. J Immunol. 134:1487–1492
Author information
Authors and Affiliations
Additional information
Offprint requests to: H. C. Hoogsteden.
Rights and permissions
About this article
Cite this article
Hoogsteden, H.C., van Ha, P.T.W., Wijkhuijs, J.M. et al. Differences in expression of monocyte/macrophage surface antigens in peripheral blood and bronchoalveolar lavage cells in interstitial lung diseases. Lung 171, 149–160 (1993). https://doi.org/10.1007/BF00183944
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00183944