Abstract
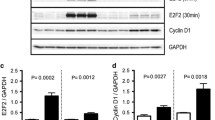
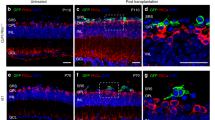
• Background: Intraocular transplantation of genetically modified cells that release a particular substance could have a major impact on the treatment of various ocular diseases. We studied the expression of the reporter gene β-galactosidase (lacZ) in transplanted retinal pigment epithelial (RPE) cells in vivo • Methods: RPE cells from pigmented rabbits were transduced with the β-galactosidase gene in a retroviral vector. Cells were then assayed for gene expression and transplanted subretinally into the eyes of New Zealand White rabbits. RPE cells that were transduced with a similar vector without the β-galactosidase gene were used as controls. Rabbits were killed on days 1, 7, and 21 and the eyes processed for transmission electron microscopy • Results: Neomycin-resistant rabbit RPE cells that showed β-galactosidase activity were generated within 2–5 weeks. After transplantation, viable RPE cells that expressed the transgene and that phagocytosed rod outer segments were observed on days 1, 7, and 21 • Conclusions: The results show that generation of genetically modified RPE cells is feasible and that the transplanted cells remain viable and continue to express the transgene in the subretinal space of the host animal for at least 21 days. Transplantation of such genetically modified RPE cells could provide a new tool for studying retinal diseases and, potentially, for correcting metabolic abnormalities in retinal degenerations and dystrophies.
Similar content being viewed by others
References
Behbehani MM, Bowyer DW, Ruffolo JJ, Kranias G (1984) Preservation of retinal function in the RCS rat by laser treatment. Retina 4:257–263
Bennett J, Wilson J, Sun D, Forbes B, Maguire A (1994) Adenovirus vector-mediated in vivo gene transfer into adult murine retina. Invest Ophthalmol Vis Sci 35:2535–2542
Cone RD, Mulligan RC (1992) High-efficiency gene transfer into mammalian cells: generation of helper-free recombinant retrovirus with broad mammalian host range. Biotechnology 24:420–424
Cornetta K, Anderson WF (1989) Protamine sulfate as an effective alternative to polybrene in retrovirul-mediated gene transfer: implications for human gene therapy. J Virol Methods 23:187–194
el Dirini AA, Wang HM, Ogden TE, Ryan SJ (1992) Retinal pigment epithelium implantation in the rabbit: technique and morphology. Graefe's Arch Clin Exp Ophthalmol 230:292–300
Etienne-Julan M, Roux P, Carillo S, Jeanteur P, Piechaczyk M (1992) The efficiency of cell targeting by recombinant retroviruses depends on the nature of the receptor and the composition of the artificial cell-virus linker. J Gen Virol 73:3251–3255
Faktorovich EG, Steinberg RH, Yasumura D, Matthes MT, LaVail MM (1990) Photoreceptor degeneration in inherited retinal dystrophy delayed by basic fibroblast growth factor. Nature 347:83–86
Fisher LJ, Jinnah HA, Kale LC, Higgins GA, Gage FH (1991) Survival and function of intrastriatally grafted primary fibroblasts genetically modified to produce L-dopa. Neuron 6:371–380
Gage FH, Rosenberg MB, Tuszynski MH, Yoshida K, Armstrong DM, Hayes RC, Friedmann T (1990) Gene therapy in the CNS: intracerebral grafting of genetically modified cells. Prog Brain Res 86:205–217
Gouras P, Lopez R, Brittis M, Kjeldbye H (1992) The ultrastructure of transplanted rabbit retinal epithelium. Graefe's Arch Clin Exp Ophthalmol 230:468–475
Kaleko M, Garcia JV, Miller AD (1991) Persistent gene expression after retroviral gene transfer into liver cells in vivo. Hum Gene Ther 2:27–32
LaVail MM, Li L, Turner JE, Yasumura D (1992) Retinal pigment epithelial cell transplantation in RCS rats: normal metabolism in rescued photoreceptors. Exp Eye Res 55:555–562
LaVail MM, Unoki K, Yasumura D, Matthes MT, Yancopoulos G, Steinberg RH (1992) Multiple growth factors, cytokines, and neurotrophins rescue photoreceptors from the damaging effects of constant light. Proc Natl Acad Sci USA 89:11249–11253
Li T, Adamian M, Roof DJ, Berson EL, Dryja TP, Roessler BJ, Davidson BL (1994) In vivo transfer of a reporter gene to the retina mediated by an adenoviral vector. Invest Ophthalmol Vis Sci 35:2543–2549
McLachlin JR, Mittereder N, Daucher MB, Kadan M, Eglitis MA (1993) Factors affecting retroviral vector function and structural integrity. Virology 195:1–5
Miller AD, Miller DG, Garcia JV, Lynch CM (1993) Use of retroviral vectors for gene transfer and expression. Methods Enzymol 217:581–599
Miller DG, Adam MA, Miller AD (1990) Gene transfer by retrovirus vectors occurs only in cells that are actively replicating at the time of infection. Mot Cell Biol 10:4239–4242 [erratum: Mol Cell Biol (1992) 12:433]
Murnane JP, Yezzi MJ, Young BR (1990) Recombination events during integration of transfected DNA into normal human cells. Nucleic Acids Res 18:2733–2738
Palmer TD, Rosman GJ, Osborne WRA, Miller AD (1991) Genetically modified skin fibroblasts persist long after transplantation but gradually inactivate introduced genes. Proc Natl Acad Sci USA 88:1330–1334
Peyman GA, Blinder KJ, Paris CL, Alturki W, Nelson NC Jr, Desai U (1991) A technique for retinal pigment epithelium transplantation for age-related macular degeneration secondary to extensive subfoveal scarring. Ophthalmic Surg 22:102–108
Rettinger SD, Kennedy SC, Wu X, Saylors RL, Hafenrichter DG, Flye MW, Ponder KP (1994) Liver-directed gene therapy: quantitative evaluation of promoter elements by using in vivo retroviral transduction. Proc Natl Acad Sci USA 91:1460–1464
Rousculp MD, Goldsmith KT, Garver RI Jr (1992) Quantitative evaluation of retroviral gene transduction efficiency in human lung cancer cells. Hum Gene Ther 3:471–477
Schuschereba ST, Bowman PD, Ferrando RE, Lund DJ, Quong JA, Vargas JA (1994) Accelerated healing of laser-injured rabbit retina by basic fibroblast growth factor. Invest Ophthalmol Vis Sci 35:945–954
Seaton AD, Turner JE (1992) RPE transplants stabilize retinal vasculature and prevent neovascularization in the RCS rat. Invest Ophthalmol Vis Sci 33:83–91
Silverman MS, Hughes SE (1990) PatPhotoreceptor rescue in the RCS rat without pigment epithelium transplantation. Curr Eye Res 9:183–191
Wongpichedchai S, Weiter JJ, Weber P, Dorey CK (1992) Comparison of external and internal approaches for transplantation of autologous retinal pigment epithelium. Invest Ophthalmol Vis Sci 33:3341–3352
Yamaguchi K, Yamaguchi K, Young RW, Gaur VP, Greven CM, Slusher MM, Turner JE (1992) Vitreoretinal surgical technique for transplanting retinal pigment epithelium in rabbit retina. Jpn J Ophthalmol 36:142–150
Ye J, Wang HM, Ogden TE, Ryan SJ (1993) Allotransplantation of rabbit retinal pigment epithelial cells double-labelled with 5-bromodeoxyuridine (BrdU) and natural pigment. Curr Eye Res 12:629–639
Zinn KM, Benjamin-Henkind JV (1979) Anatomy of human retinal epithelium. In: Zinn KM, Marmor MF (eds) The retinal pigment epithelium. Harvard University Press, Cambridge, Mass, pp 3–31
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Osusky, R., Jiang, M., Spee, C. et al. β-Galactosidase transgene expression in transplanted rabbit retinal pigment epithelial cells in vivo. Graefe's Arch Clin Exp Ophthalmol 233, 220–225 (1995). https://doi.org/10.1007/BF00183595
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00183595