Summary
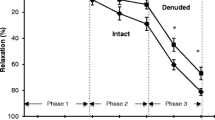
The influence of endothelium-derived nitric oxide (EDNO) on relaxation induced by the nitrovasodilators, sodium nitroprusside and sodium nitrite was assessed in phenylephrine-stimulated hamster thoracic aortas, a preparation that displays significant basal release of EDNO. Removal of the endothelium or treatment with the NO synthase inhibitors, NG-nitro-l-arginine (L-NAG, 10–30 µM) or NG-methyl-l-arginine (L-NMMA; 100 µM) increased the potency and, except for sodium nitroprusside in endothelium-denuded segments, also increased the efficacy of the nitrovasodilators. Removal of the endothelium had no effect on relaxations induced by isoproterenol, an indication that these effects were specific for the nitrovasodilators. Removal of the endothelium, treatment of endothelium-intact preparations with L-NAG or L-NMMA, or exposure of these vessels to the guanylate cyclase inhibitor, methylene blue (10 µM) increased reactivity of the aortas to the guanosine 3′:5′-cyclic monophosphate (cGMP) analogue, 8-Br cGMP. Measurement of cGMP revealed that endothelium-intact segments had a 6.5 fold higher level of cGMP than endothelium-denuded preparations and that sodium nitroprusside increased cGMP in both preparations by similar amounts in a concentration-dependent fashion. Exposure of endothelium-denuded or L-NAG-treated segments to sodium nitroprusside, to mimic the effects of basally released EDNO, depressed sodium nitrite and 8-Br cGMP reactivity in a manner similar to endothelium-intact segments. These data indicate that EDNO increases cGMP levels in vascular smooth muscle and that the elevated cGMP levels depress nitrovasodilator and 8-Br cGMP reactivities.
Similar content being viewed by others
References
Alheid A, Dudel C, Förstermann U (1987) Selective inhibition by gossypol of endothelium-dependent relaxations augments relaxations to glyceryl trinitrate in rabbit coeliac artery. Br J Pharmacol 92:237–240
Busse R, Pohl U, Mülsch A, Bassenge E (1989) Modulation of the vasodilator action of SIN-1 by the endothelium. J Cardiovasc Pharmacol 14 [Suppl]:S81-S85
Dinerman JL, Lawson DL, Mehta JL (1991) Interactions between nitroglycerin and endothelium in vascular smooth muscle relaxation. Am J Physiol 260:H698-H701
Feelisch M, Noack E (1987) Nitric oxide (NO) formation from nitrovasodilators occurs independently of hemoglobin or nonheme iron. Eur J Pharmacol 142:465–469
Furchgott RF, Carvalho MHC, Khan MT, Matsunaga K (1987) Evidence for endothelium-dependent vasodilation of resistance vessels by acetylcholine. Blood Vessels 24:145–149
Förstermann U, Trogisch G, Busse R (1984) Species-dependent differences in the nature of endothelium-derived vascular relaxing factor. Eur J Pharmacol 106:639–643
Ignarro LJ, Kadowitz PJ (1985) The pharmacological and physiological role of cyclic GMP in vascular smooth muscle relaxation. Ann Rev Pharmacol Toxicol 25:171–191
Jackson WF (1987) Species differences in the effects of inhibitors of endothelium-dependent relaxation. Fed Proc 46:827 (abstr)
Jackson WF (1988) Oscillations in active tension in hamster aortas: role of the endothelium. Blood Vessels 25:144–156
Jackson WF (1989) The endothelium-derived relaxing factor. J Reconstruc Microsurg 5:263–271
Jackson WF, Mülsch A, Busse R (1991) Rhythmic smooth muscle activity in hamster aortas is mediated by continuous release of nitric oxide from the endothelium. Am J Physiol 260:H248-H253
Kramer GL, Hardman JG (1980) Cyclic nucleotides and blood vessel contraction. In: Bohr DF, Somlyo AP, Sparks HV (eds) Handbook of physiology, sect 2: The cardiovascular system, volume 11, vascular smooth muscle. American Physiological Society, Bethesda, pp 179–199
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with phenol reagent. J Biol Chem 193:265–275
Moncada S, Rees DD, Schultz R, Palmer RMJ (1991) Development and mechanism of a specific supersensitivity to nitrovasodilators after inhibition of vascular nitric oxide synthesis in vivo. Proc Natl Acad Sci USA 88:2166–2170
Mülsch A, Busse R (1990) NG-nitro-l-arginine (N5-[imino(nitro-amino)methyl]-l-ornithine) impairs endothelium-dependent dilations by inhibiting cytosolic nitric oxide synthesis from l-arginine. Naunyn Schmiedeberg's Arch Pharmacol 341:143–147
Ott L (1990) An introduction to statistical methods and data analysis, 3rd edn. PWS-Kent, Boston
Palmer RMJ, Ferrige AG, Moncada S (1987) Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature 327:524–526
Palmer RMJ, Ashton DS, Moncada S (1988a) Vascular endothelial cells synthesize nitric oxide from l-arginine. Nature 333:664–666
Palmer RMJ, Rees DD, Ashton DS, Moncada S (1988b) l-arginine is the physiological precursor of nitric oxide in endothelium-dependent relaxation. Biochem Biophys Res Commun 153: 1251–1256
Pohl U, Busse R (1987) Endothelium-derived relaxant factor inhibits effects of nitrocompounds in isolated arteries. Am J Physiol 252:H307-H313
Rapoport RM, Draznin MB, Murad F (1983) Endothelium-dependent and nitrovasodilator-induced relaxation may be mediated through cyclic GMP formation and cGMP-dependent protein phosphorylation. Trans Assoc Am Phys 96:19–30
Saeed M, Bing R (1986) Endothelial derived relaxing factor and the effect of 8-bromo cyclic GMP on coronary arteries in perfused and in vivo rabbit hearts. J Mol Cell Cardiol 18 [Suppl]:5037
Sellke FW, Myers PR, Bates JN, Harrison DG (1990) Influence of vessel size on the sensitivity of porcine coronary microvessels to nitroglycerin. Am J Physiol 258:H515-H520
Shirasaki Y, Su C (1985) Endothelium removal augments vasodilation by sodium nitroprusside and sodium nitrite. Eur J Pharmacol 114:93–96
Shirasaki Y, Su C, Lee TJF, Kohn P, Cline WHJR, Nickols GA (1986) Endothelial modulation of vascular relaxation to nitrovasodilators in aging and hypertension. J Pharm Exp Ther 239:861–866
Waldman SA, Murad F (1987) Cyclic GMP synthesis and function. Pharmacol Rev 39:163–196
Waud DR (1975) Analysis of dose-response curves. In: Daniel EE, Paton DM (eds) Methods in pharmacology, vol 3. Smooth muscle. Plenum Press, New York, pp 471–506
Author information
Authors and Affiliations
Additional information
Send offprint requests to W. F. Jackson at the above address
Rights and permissions
About this article
Cite this article
Jackson, W.F., Busse, R. Elevated guanosine 3′:5′-cyclic monophosphate mediates the depression of nitrovasodilator reactivity in endothelium-intact blood vessels. Naunyn-Schmiedeberg's Arch Pharmacol 344, 345–350 (1991). https://doi.org/10.1007/BF00183010
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00183010