Abstract
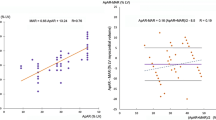
This prospective study in 42 patients with chronic coronary artery disease and severe wall motion abnormalities (sWMA) on cineventriculography (24 patients with previous myocardial infarction; ejection fraction, 45%±13%) was designed to compare myocardial thallium-201 uptake after rest injection and normalized fluorodeoxyglucose (18FDG) uptake (after oral glucose load) for assessment of a rest 201Tl protocol to evaluate myocardial viability. The left ventricle was divided into the supply territory of the left anterior descending coronary artery (LAD) and the lateral wall and posterior territory (inferior, posterior and posteroseptal segments) because of the high variability of left circumflex and right coronary artery supply territories. Segmental 201Tl uptake in single-photon emission tomography (SPET) and segmental normalized 18FDG uptake (13 segments per patient) showed a close linear relationship in the LAD territory (r=0.79) and in the lateral wall (r=0.77), while the correlation in the posterior territory was considerably lower (r=0.52). 201Tl/18FDG concordance was defined as an 18FDG uptake exceeding 201Tl uptake by < 20%. Discordance was assumed if 18FDG exceeded 201Tl uptake by at least 20%. Concordant results were shown by 81% (439/541) of segments. In segments with severe 201Tl reduction (≤ 50% of peak, n=78) discordance was observed in 10% of segments in the LAD territory and lateral wall (n=62) and in 44% of segments in the posterior territory (n=16). In segments with moderate 201Tl reduction (51%−75%, n=205) discordance occured in 12% (LAD and lateral wall, n=126) or 46% (posterior territory, n=79) of segments, respectively. Severe defects were defined as the entire area with 201Tl uptake ≤50% within a defined territory. Discordance was observed in 6/43 (14%) of these. Of 90 areas with sWMA on cineventriculography, 12 showed discordant results. Ten of these 12 discordant areas affected septum or posterior wall. In areas with normal wall motion or only mild hypokinesis, discordance occured in the septum or posterior wall in 22% whereas the figure for the anterior or lateral wall was only 2%. These results point to a significant role of photon attenuation in 201Tl SPET imaging in the septum and posterior wall. It is concluded that 201Tl SPET using a rest protocol identifies viable myocardium in the supply area of the LAD and in the lateral wall with high accuracy compared to 18FDG positron emission tomography while disordance in the posterior territory may be governed by photon attenuation in the SPET study rather than by a pathophysiological difference.
Similar content being viewed by others
References
Rigo P, Bailey IK, Griffith LSC, et al. Stress thallium-201 myocardial scintigraphy for the detection of individual coronary arterial lesions in patients with and without previous myocardial infarction. Am J Cardiol 1981;48: 209–216.
Buell U, Stirner H, vom Dahl J, et al. Quantitative evaluation of myocardial stress/rest 201Tl SPET: results of a ROI-based method in 108 patients with CAD. Nucl Med 1987;26: 234–240.
Pohost GM, Zir LM, Moore RM, et al. Differentiation of transiently ischemic from infarcted myocardium by serial imaging after a single dose of thallium 201. Circulation 1979;55: 294–302.
Hör G, Sebening H, Sauer E, et al. 201-Tl redistribution analysis in early and delayed myocardial scintigrams of patients with coronary heart disease. Eur J Nucl Med 1979;4: 343–350.
Gibson RS, Watson DD, Taylor GJ, et al. Prospective assessment of regional myocardial perfusion before and after coronary revascularization surgery by quantitative thallium-201 scintigraphy. J Am Coll Cardiol 1983;1: 804–815.
Liu P, Kiess MC, Okada RD, et al. The persistent defect on exercise thallium imaging and its fate after myocardial revascularization: does it represent scar or ischemia? Am Heart J 1985;110: 996–1001.
Kiat H, Berman DS, Maddahi J, et al. Late reversibility of tomographic myocardial thallium-201 defects: an accurate marker of myocardial viability. J Am Coll Cardiol 1988;12: 1456–1463.
Yang LD, Berman DS, Kiat H, Resser KJ, Freedman JD, Royanski A, Maddahi JM. The frequency of late reversibility in SPET thallium-201 stress-redistribution studies. J Am Coll Cardiol 1990;15: 334–340.
Dilsizian V Rocco TP, Freedman NMT, Leon MB, Bonow RO. Enhanced detection of ischemic but viable myocardium by the reinjection of thallium after stress-redistribution imaging. N Engl J Med 1990;323: 141–146.
Ohtani H, Tamaki N, Yonekura Y, et al. Value of thallium-201 reinjection after delayed SPET imaging for predicting reversible ischemia after coronary artery bypass grafting. Am J Cardiol 1990;66: 394–399.
Brunken R, Schwaiger M, Grover-McKay M, Phelps ME, Tillisch J, Schelbert HR (1987) Positron emission tomography detects tissue metabolic activity in myocardial segments with persistent thallium perfusion defects. J Am Coll Cardiol 10: 557–567.
Brunken R, Kottou S, Nienaber CA, Schwaiger M, Ratib OM, Phelps ME, Schelbert HR (1989) PET detection of viable tissue in myocardial segments with persistent defects at Tl-201 SPET. Radiology 65: 65–73.
Tamaki N, Yonekura Y, Yamashita K, et al. Relation of left ventricular perfusion and wall motion with metabolic activity in persistent defects on thallium-201 tomography in healed myocardial infarction. Am J Cardiol 1988; 62: 202–208.
Altehoefer C, Kaiser HJ, Doerr, Feinendegen C, Beilin I, Uebis R, Buell U. Fluorine-18 deoxyglucose PET for assessment of viable myocardium in perfusion defects in 99mTc-MIBI SPET: a comparative study in patients with coronary artery disease. Eur J Nucl Med 1992;19: 334–342.
Segall GM, Davis MJ. Prone versus supine thallium myocardial SPET: a method to decrease artifactual inferior wall defects. J Nucl Med 1989;30: 548–555.
Marshall RC, Tillisch JH, Phelps ME, Huang SC, Carson R, Henze E, Schelbert HR. Identification and differentiation of resting myocardial ischemia and infarction in man with positron computed tomography, 18-F labeled fluorodeoxyglucose and N-13 ammonia. Circulation 1983;67: 766–778.
Tillisch J, Brunken R, Marshall R, Schwaiger M, Mandelkern M, Phelps M, Schelbert H (1986) Reversibility of cardiac wall motion abnormalities predicted by positron tomography. N Engl J Med 314;884–888.
Tamaki N, Yoshihara Y, Yamashita K, et al. Positron emission tomography using fluorine-18 deoxyglucose in evaluation of coronary bypass grafting. Am J Cardiol 1989;64: 860–865.
Schwaiger M, Hicks R. The clinical role of metabolic imaging of the heart by positron emission tomography. J Nucl Med 1991;32: 565–578.
Dilsizian V Freedman NMT, Bacharach SL, Perrone-Filardi P, Bonow RO. Regional thallium uptake in irreversible defects. Magnitude of change in thallium activity after reinjection distinguishes viable from nonviable myocardium. Circulation 1992;85: 627–634.
Braunwald E, Rutherford JD (1986) Reversible ischemic left ventricular dysfunction: evidence for “hibernating” myocardium. J Am Coll Cardiol 8: 1476–1470.
Brundage BH, Massie BM, Botvinik EH (1984) Improved regional ventricular function after successful surgical revascularization. J Am Coll Cardiol 3: 902–908.
Bonow RO, Dilsizian V, Cuocolo A, Bachaxach SL. Identification of viable myocardium in patients with chronic coronary artery disease and left ventricular dysfunction. Comparison of thallium scintigraphy with reinjection and PET imaging with 18F-fluorodeoxyglucose. Circulation 1991;83: 26–37.
Tamaki N, Ohtani H, Yamashita K, et al. Metabolic activity of new fill-in after thallium-201 reinjection: comparison with positron emission tomography using fluorine-18-deoxyglucose. J Nucl Med 1991;32: 673–678.
Dilsizian V, Bonow RO. Differential uptake and apparent 201Tl washout after thallium reinjection. Options regarding early redistribution imaging before reinjection or late redistribution imaging after reinjection. Circulation 1992;85: 1032–1038.
Forman R, Kirk ES. Thallium-201 accumulation during reperfusion of ischemic myocardium: dependence on regional blood flow rather than viability. Am J Cardiol 1984;54: 659–663.
Sorenson JA, Phelps ME (1987) Physics in nuclear medicine. Philadelphia: Saunders.
Strauer BE, Buerger S, Buell U. Multifactorial determination of 201-thallium uptake of the heart: an experimental study concerning the influence of ventricular mass, perfusion and oxygen consumption. Basic Res Cardiol 1978;73: 298–306.
Berger BC, Watson DD, Burwell LR, Crosby IK, Wellons HA, Teates CD, Beller GA. Redistribution of thallium at rest in patients with stable and unstable angina and the effect of coronary artery bypass surgery. Circulation 1979;60: 1114–1125.
Gewirtz H, Beller GA, Strauss HW, Dinsmore RE, Zir LM, McKusick KA, Pohost GM. Transient defects of resting thallium scans in patients with coronary artery disease. Circulation 1979;59: 707–713.
Iskandrian AS, Hakki A, Kane SA, Goel IP, Mundth ED, Hakki A, Segal BL. Rest and redistribution thallium-201 myocardial scintigraphy to predict improvement in left ventricular function after coronary arterial bypass grafting. Am J Cardiol 1983;51: 1312–1316.
Knuuti MJ, Nuutila P, Ruotsalainen U et al. Euglycemic hyperinsulinemic clamp and oral glucose load in stimulating myocardial glucose utilization during positron emission tomography. J Nucl Med 1992;33: 1255–1262.
Neely JR, Morgan HE. Relationship between carbohydrate and lipid metabolism and the energy balance of the heart muscle. Ann rev Physiol 1974;36: 413–459.
Gropler RJ, Siegel BA, Lee KJ et al. Nonuniformity in myocardial accumulation of fluorine- l8-fluorodeoxyglucose in normal fasted humans. J Nucl Med 1990;31: 1749–1756.
Liedke JA. Alterations of carbohydrate and lipid metabolism in the acutely ischemic heart. Prog Cardiovasc Dis 1981;23: 321–336.
Camici P, Araujo LI, Spinks T, et al. Increased uptake of 18F-fluorodeoxyglucose in postischemic myocardium of patients with exercise-induced angina. Circulation 1986;74: 81–88.
vom Dahl J, Altehoefer C, Sheehan FH, et al. Recovery of myocardial function following coronary revascularization: impact of viability and long-term vessel patency as assessed by preoperative F-18 FDG PET and serial angiography. J Nucl Med 1993;23P.
Author information
Authors and Affiliations
Additional information
Correspondence to: C. Aftehoefer
Rights and permissions
About this article
Cite this article
Altehoeter, C., vom Dahl, J., Buell, U. et al. Comparison of thallium-201 single-photon emission tomography after rest injection and fluorodeoxyglucose positron emission tomography for assessment of myocardial viability in patients with chronic coronary artery disease. Eur J Nucl Med 21, 37–45 (1994). https://doi.org/10.1007/BF00182304
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00182304