Abstract
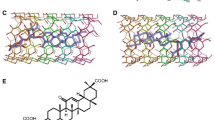
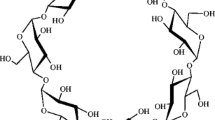
Cyclodextrins are cyclic oligosaccharides known for their ability to include substrate molecules in their hydrophobic cavity. Moreover, cyclodextrins show a hemolytic activity when mm concentrations are added to blood. This hemolysis is commonly interpreted as a massive dissociation of phospholipids from the cell membrane due to the formation of complexes with the cyclodextrins. In the literature, a complexation between α-cyclodextrin (α CD) and phosphatidylinositol (PI) specific to the inositol headgroup has been proposed. But the need for the detailed interaction mechanism between the two molecules motivated the present work based on molecular dynamics simulations. Investigation of long range electrostatic interactions shows that a mutual approach of the molecules is only possible when the primary hydroxyl side of α CD faces the inositol headgroup of PI. This orientation is also the most favourable from adiabatic- and free-energy profiles calculated along a reaction coordinate that leads to an inclusion of PI into a CD. For free energy simulations, partial hydration of the model has been used. A study of glycosidic bond dihedral angles in α CD shows an increase in dihedral fluctuations before complexation and a dihedral “freezing” once the complex is formed.
Similar content being viewed by others
References
Bergeron RJ, Charming MA, McGovern KA (1978) Dependence of cycloamylose-substrate binding on charge. J Am Chem Soc 100:2878–2883
Berne BJ, Borkovec M, Straub JE (1988) Classical and modern methods in reaction rate theory. J Phys Chem 92: 3711–3725
Brooks CL, Karplus M (1983) Deformable stochastic boundaries in molecular dynamics. J Chem Phys 79:6312–6325
Brooks BR, Bruccoleri RE, Olafson BD, States DJ, Swaminathan S, Karplus M (1983) CHARMM: a program for macromolecular energy, minimization, and dynamics calculations. J Comp Chem 4:187–217
Cramer F, Saenger W Spatz HC (1967) Inclusion compounds. XIX. The formation of inclusion compounds of α-cyclodextrin in aqueous solutions. Thermodynamics and kinetics. J Am Chem Soc 89:14–20
Crouzy S, Woolf TB, Roux B (1994) A molecular dynamics study of gating in dioxolane-linked gramicidin A channels. Biophys J 67: 1370–1386
Crouzy S, Fauvelle F, Debouzy JC, Göschl M, Chapron Y (1996) Investigation of the α-cyclodextrin-myo-inositol phosphate inclusion complex by NMR spectroscopy and molecular modeling. Carbohydr Res (in press)
Dewar M, Thiel W (1977) Ground states of molecules 38. The MNDO method. approximations and parameters. J Am Chem Soc 99: 4899–4907
Elder M, Hitchcock P, Mason R, Shipley GG (1977) A refinement analysis of the cristallography of the phospholipid, 1,2-dilauroylDL-phosphatidylethanolamine and some remarks on lipid-lipid and lipid-protein interactions. Proc R Soc Lond A 354: 157–170
Fauvelle F, Debouzy JC, Nardin R, Gadelle A (1994) Nuclear magnetic resonance study of a polar headgroup determined α-cyclodextrin-phospholipid association. Bioelectrochem Bioenerg 33:95–99
Gelb RI, Schwartz LM, Johnson RF, Laufer DA (1979) The complexation chemistry of cyclohexaamylose. 4. Reactions of cyclohexaamylose with formic, acetic, and benzoic acids and their conjugate bases. J Am Chem Soc 101: 1869–1874
Hansbro PM, Byard SJ, Bushby RJ, Turnbull PJH, Boden N, Saunders MR, Novelli R, Reid DG (1992) The conformational behaviour of phosphatidylinositol in model membranes: H-NMR studies. Biochim Biophys Acta 1112: 187–196
Hingerty B, Saenger W (1975) Disorder in a hydrophobic cage illustrated by X-ray structure of α-cyclodextrin-methanol pentahydrate adduct. Nature 255: 396–397
Irie T, Otagiri M, Sunada M, Uekama K, Ohtani Y, Yamada Y, Sugiyama Y (1982) Cyclodextrin induced hemolysis and shape changes of human erythrocytes in vitro. J Pharm Dyn 5: 741–744
Jorgensen WL, Chandrasekhar J, Madura JD, Impey RW, Klein ML (1983) Comparison of simple potential functions for simulating liquid water. J Chem Phys 79:926–935
Koehler JEH, Saenger W, van Gunsteren WF (1987) A molecular dynamics simulation of crystalline α-cyclodextrin hexahydrate. Eur Biophys J 15: 197–210
Koehler JEH, Saenger W, van Gunsteren WF (1988) Conformational differences between α-cyclodextrin in aqueous solution and in crystalline form. A molecular dynamics study. J Mol Biol 203:241–250
Kramers HA (1940) Brownian motion in a field of force and the diffusion model of chemical reactions. Physica 7: 284–304
Kraulis PJ (1991) A program to produce both detailed and schematic plots of protein structures. J Appl Cryst 24:946–950
Manor PC, Saenger W (1974) Topography of cyclodextrin Inclusion complexes. III. Crystal and molecular structure of cyclohexaamylose hexahydrate and the (H2O)2 inclusion complex. J Am Chem Soc 96:3630–3639
Myles AMC, Barlow DJ, France G, Lawrence MJ (1994) Analysis and modelling of the structures of β-cyclodextrin complexes. Biochim Biophys Acta 1199:27–36
Némethy G, Scheraga HA (1962) Structure of water and hydrophobic bonding in proteins. II. model for the thermodynamic properties of aqueous solutions of hydrocarbons. J Chem Phys 36: 3401–3417
Ohtani Y, Irie T, Uekama K, Fukunaga K, Pitha J (1989) Differential effects of β- and γ-cyclodextrins in human erythrocytes. Eur J Biochim 186:17–22
Patey GN, Valleau JP (1975) A Monte Carlo method for obtaining the interionic potential of mean force in ionic solution. J Chem Phys 63:2334–2339
Rabinowitz IN, Kraut J (1964) The crystal structure of myo-inositol. Acta Cryst 17:159–168
Saenger W, Noltemeyer M, Manor PC, Hingerty B, Klar B (1976) “induced-fit”-type complex formation of the model enzyme α-cyclodextrin. Bioorg Chem 5: 187–195
Saenger W (1980) Cyclodextrin inclusion compounds in research and industry. Angew Chem Int Ed Engl 19:344–362
Szejtli J, Cserhati T, Szogyi M (1986) Interactions between cyclodextrin and cell-membrane phospholipids. Carbohydr Polym 6: 35–49
Tabushi I, Kiyosuke Y, Sugimoto T, Yamamura K (1978) Approach to the aspects of driving force of inclusion by α-cyclodextrin. J Am Chem Soc 100:916–919
Takusagawa F, Jacobson RA (1978) The crystal and molecular structure of α-maltose. Acta Cryst B 34:213–218
Uekama K, Hirayama F, Matsuo N, Koinuma H (1978) Structural elucidation of the inclusion complex of tolbutamine with α- and β-cyclodextrins in aqueous solution. Chem Lett, pp 703–706
van Gunsteren WF, Berendsen HJC (1977) Algorithms for macromolecular dynamics and constraint dynamics. Mol Phys 34: 1311–1327
Wood DJ, Hruska FE, Saenger W (1977) 1H NMR study of the inclusion of aromatic molecules in α-cyclodextrin. J Am Chem Soc 99:1735–1740
Woolf TB, Roux B (1994) The conformational flexibility of o-phosphorylcholine and o-phosphorylethanolamine: A molecular dynamics study of solvation effects. J Am Chem Soc 116:5916–5926
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Göschl, M., Crouzy, S. & Chapron, Y. Molecular dynamics study of an α-cyclodextrin-phosphatidylinositol inclusion complex. Eur Biophys J 24, 300–310 (1996). https://doi.org/10.1007/BF00180371
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00180371